当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanism‐Guided Engineering of ω‐Transaminase to Accelerate Reductive Amination of Ketones
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2015-05-13 , DOI: 10.1002/adsc.201500211 Sang-Woo Han , Eul-Soo Park , Joo-Young Dong , Jong-Shik Shin
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2015-05-13 , DOI: 10.1002/adsc.201500211 Sang-Woo Han , Eul-Soo Park , Joo-Young Dong , Jong-Shik Shin
|
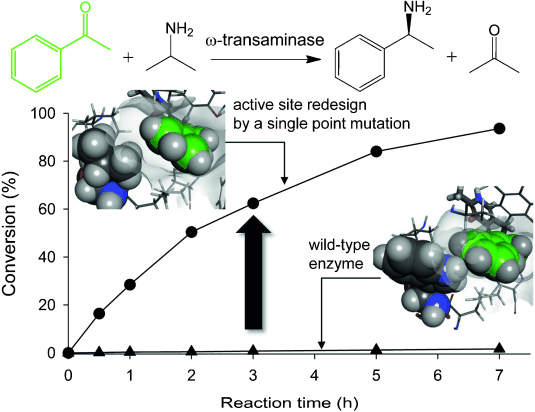
Asymmetric reductive amination of ketones using ω‐transaminases (ω‐TAs) offers a promising alternative to the chemocatalytic synthesis of chiral amines. One fundamental challenge to the biocatalytic strategy is the very low enzyme activities for most ketones compared with native substrates (i.e., <1% relative to pyruvate). Here we have demonstrated that a single point mutation in the active site of the (S)‐selective ω‐TA from Ochrobactrum anthropi could induce a remarkable acceleration of the amination reaction without any loss in stereoselectivity and enzyme stability. Molecular modeling of quinonoid intermediates, alanine scanning mutagenesis and kinetic analysis revealed that the W58 residue acted as a steric barrier to binding and catalytic turnover of ketone substrates. Removal of the steric strain by W58L substitution, which was selected by partial saturation mutagenesis, led to dramatic activity improvements for structurally diverse ketones (e.g., 340‐fold increase in kcat/KM for acetophenone). The W58L mutant afforded an efficient synthesis of enantiopure amines (i.e., >99% ee) using isopropylamine as an amino donor.
中文翻译:

ω-转氨酶的机制指导工程可加速酮的还原胺化
使用ω-转氨酶(ω-TAs)对酮进行不对称还原胺化为手性胺的化学催化合成提供了有希望的替代方法。生物催化策略的一个基本挑战是与天然底物相比,大多数酮的酶活性非常低(即,相对于丙酮酸<1%)。在这里,我们证明了人O骨(S)选择性ω-TA的活性位点发生单点突变可以诱导胺化反应显着加速,而不会损失立体选择性和酶稳定性。醌类中间体的分子模型,丙氨酸扫描诱变和动力学分析表明,W58残基充当对酮底物的结合和催化周转的空间屏障。通过部分饱和诱变选择的W58L替代去除位阻菌株,可显着提高结构上多样化的酮的活性(例如,苯乙酮的k cat / K M升高340倍)。W58L突变体提供了使用异丙胺作为氨基供体的对映纯胺(即> 99%ee)的有效合成方法。
更新日期:2015-05-13
中文翻译:

ω-转氨酶的机制指导工程可加速酮的还原胺化
使用ω-转氨酶(ω-TAs)对酮进行不对称还原胺化为手性胺的化学催化合成提供了有希望的替代方法。生物催化策略的一个基本挑战是与天然底物相比,大多数酮的酶活性非常低(即,相对于丙酮酸<1%)。在这里,我们证明了人O骨(S)选择性ω-TA的活性位点发生单点突变可以诱导胺化反应显着加速,而不会损失立体选择性和酶稳定性。醌类中间体的分子模型,丙氨酸扫描诱变和动力学分析表明,W58残基充当对酮底物的结合和催化周转的空间屏障。通过部分饱和诱变选择的W58L替代去除位阻菌株,可显着提高结构上多样化的酮的活性(例如,苯乙酮的k cat / K M升高340倍)。W58L突变体提供了使用异丙胺作为氨基供体的对映纯胺(即> 99%ee)的有效合成方法。

































 京公网安备 11010802027423号
京公网安备 11010802027423号