当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Molecular mechanism for USP7-mediated DNMT1 stabilization by acetylation.
Nature Communications ( IF 14.7 ) Pub Date : 2015-May-11 , DOI: 10.1038/ncomms8023
Jingdong Cheng , Huirong Yang , Jian Fang , Lixiang Ma , Rui Gong , Ping Wang , Ze Li , Yanhui Xu
Nature Communications ( IF 14.7 ) Pub Date : 2015-May-11 , DOI: 10.1038/ncomms8023
Jingdong Cheng , Huirong Yang , Jian Fang , Lixiang Ma , Rui Gong , Ping Wang , Ze Li , Yanhui Xu
|
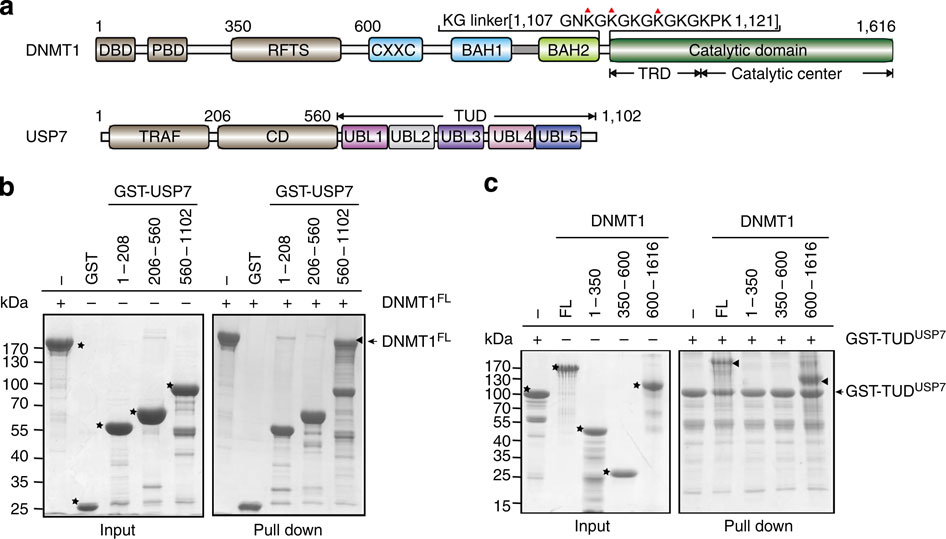
DNMT1 is an important epigenetic regulator that plays a key role in the maintenance of DNA methylation. Here we determined the crystal structure of DNMT1 in complex with USP7 at 2.9 Å resolution. The interaction between the two proteins is primarily mediated by an acidic pocket in USP7 and Lysine residues within DNMT1's KG linker. This intermolecular interaction is required for USP7-mediated stabilization of DNMT1. Acetylation of the KG linker Lysine residues impair DNMT1-USP7 interaction and promote the degradation of DNMT1. Treatment with HDAC inhibitors results in an increase in acetylated DNMT1 and decreased total DNMT1 protein. This negative correlation is observed in differentiated neuronal cells and pancreatic cancer cells. Our studies reveal that USP7-mediated stabilization of DNMT1 is regulated by acetylation and provide a structural basis for the design of inhibitors, targeting the DNMT1-USP7 interaction surface for therapeutic applications.
中文翻译:

USP7介导的DNMT1乙酰化稳定作用的分子机制。
DNMT1是重要的表观遗传调节剂,在维持DNA甲基化中起关键作用。在这里,我们确定了与USP7配合使用的DNMT1晶体结构,分辨率为2.9。两种蛋白质之间的相互作用主要是由USP7中的酸性囊和DNMT1的KG接头内的赖氨酸残基介导的。此分子间相互作用是USP7介导的DNMT1稳定所必需的。KG接头赖氨酸残基的乙酰化会损害DNMT1-USP7的相互作用并促进DNMT1的降解。使用HDAC抑制剂治疗会导致乙酰化DNMT1的增加,而总DNMT1的蛋白质减少。在分化的神经元细胞和胰腺癌细胞中观察到这种负相关性。
更新日期:2015-05-13
中文翻译:

USP7介导的DNMT1乙酰化稳定作用的分子机制。
DNMT1是重要的表观遗传调节剂,在维持DNA甲基化中起关键作用。在这里,我们确定了与USP7配合使用的DNMT1晶体结构,分辨率为2.9。两种蛋白质之间的相互作用主要是由USP7中的酸性囊和DNMT1的KG接头内的赖氨酸残基介导的。此分子间相互作用是USP7介导的DNMT1稳定所必需的。KG接头赖氨酸残基的乙酰化会损害DNMT1-USP7的相互作用并促进DNMT1的降解。使用HDAC抑制剂治疗会导致乙酰化DNMT1的增加,而总DNMT1的蛋白质减少。在分化的神经元细胞和胰腺癌细胞中观察到这种负相关性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号