当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Role of Domain Swapping in the Hetero-Oligomeric Cytochrome b6f Lipoprotein Complex
Biochemistry ( IF 2.9 ) Pub Date : 2015-05-12 00:00:00 , DOI: 10.1021/acs.biochem.5b00279 Rachna Agarwal 1 , S. Saif Hasan 1 , LaDonna M. Jones 2 , Jason T. Stofleth 1 , Christopher M. Ryan 3 , Julian P. Whitelegge 3 , David M. Kehoe 2 , William A. Cramer 1
Biochemistry ( IF 2.9 ) Pub Date : 2015-05-12 00:00:00 , DOI: 10.1021/acs.biochem.5b00279 Rachna Agarwal 1 , S. Saif Hasan 1 , LaDonna M. Jones 2 , Jason T. Stofleth 1 , Christopher M. Ryan 3 , Julian P. Whitelegge 3 , David M. Kehoe 2 , William A. Cramer 1
Affiliation
|
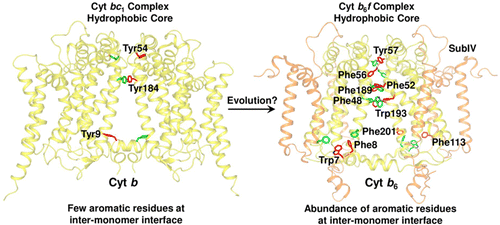
Domain swapping that contributes to the stability of biologically crucial multisubunit complexes has been implicated in protein oligomerization. In the case of membrane protein assemblies, domain swapping of the iron–sulfur protein (ISP) subunit occurs in the hetero-oligomeric cytochrome b6f and bc1 complexes, which are organized as symmetric dimers that generate the transmembrane proton electrochemical gradient utilized for ATP synthesis. In these complexes, the ISP C-terminal predominantly β-sheet extrinsic domain containing the redox-active [2Fe-2S] cluster resides on the electrochemically positive side of each monomer in the dimeric complex. This domain is bound to the membrane sector of the complex through an N-terminal transmembrane α-helix that is “swapped’ to the other monomer of the complex where it spans the complex and the membrane. Detailed analysis of the function and structure of the b6f complex isolated from the cyanobacterium Fremyella diplosiphon SF33 shows that the domain-swapped ISP structure is necessary for function but is not necessarily essential for maintenance of the dimeric structure of the complex. On the basis of crystal structures of the cytochrome complex, the stability of the cytochrome dimer is attributed to specific intermonomer protein–protein and protein–lipid hydrophobic interactions. The geometry of the domain-swapped ISP structure is proposed to be a consequence of the requirement that the anchoring helix of the ISP not perturb the heme organization or quinone channel in the conserved core of each monomer.
中文翻译:

域交换在异寡聚细胞色素b 6 f脂蛋白复合物中的作用。
有助于生物学关键的多亚基复合物稳定性的结构域交换与蛋白质寡聚化有关。对于膜蛋白装配体,异硫寡聚细胞色素b 6 f和bc 1中发生铁硫蛋白(ISP)亚基的结构域交换络合物,以对称的二聚体形式组织,产生用于ATP合成的跨膜质子电化学梯度。在这些络合物中,包含氧化还原活性[2Fe-2S]簇的ISP C端主要为β-折叠外部域位于二聚体络合物中每个单体的电化学正侧。该结构域通过“交换”到复合物的其他单体的N端跨膜α-螺旋与复合物的膜区结合,在该单体中它横跨复合物和膜。从蓝细菌弗雷米氏双歧杆菌中分离的b 6 f配合物的功能和结构的详细分析SF33表明,域交换ISP结构对于功能是必需的,但对于维护复合物的二聚体结构不是必需的。根据细胞色素复合物的晶体结构,细胞色素二聚体的稳定性归因于特定的单体蛋白-蛋白质和蛋白质-脂质疏水相互作用。提出域交换ISP结构的几何形状是由于要求ISP的锚定螺旋不干扰每个单体的保守核中的血红素组织或醌通道的结果。
更新日期:2015-05-12
中文翻译:

域交换在异寡聚细胞色素b 6 f脂蛋白复合物中的作用。
有助于生物学关键的多亚基复合物稳定性的结构域交换与蛋白质寡聚化有关。对于膜蛋白装配体,异硫寡聚细胞色素b 6 f和bc 1中发生铁硫蛋白(ISP)亚基的结构域交换络合物,以对称的二聚体形式组织,产生用于ATP合成的跨膜质子电化学梯度。在这些络合物中,包含氧化还原活性[2Fe-2S]簇的ISP C端主要为β-折叠外部域位于二聚体络合物中每个单体的电化学正侧。该结构域通过“交换”到复合物的其他单体的N端跨膜α-螺旋与复合物的膜区结合,在该单体中它横跨复合物和膜。从蓝细菌弗雷米氏双歧杆菌中分离的b 6 f配合物的功能和结构的详细分析SF33表明,域交换ISP结构对于功能是必需的,但对于维护复合物的二聚体结构不是必需的。根据细胞色素复合物的晶体结构,细胞色素二聚体的稳定性归因于特定的单体蛋白-蛋白质和蛋白质-脂质疏水相互作用。提出域交换ISP结构的几何形状是由于要求ISP的锚定螺旋不干扰每个单体的保守核中的血红素组织或醌通道的结果。































 京公网安备 11010802027423号
京公网安备 11010802027423号