当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Efficient Synthesis of Chiral Indolines using an Imine Reductase from Paenibacillus lactis
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2015-05-12 , DOI: 10.1002/adsc.201500160 Hao Li , Zheng-Jiao Luan , Gao-Wei Zheng , Jian-He Xu
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2015-05-12 , DOI: 10.1002/adsc.201500160 Hao Li , Zheng-Jiao Luan , Gao-Wei Zheng , Jian-He Xu
|
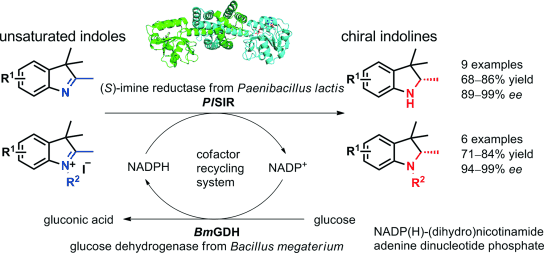
An enzymatic process for the efficient asymmetric reduction of 3H‐indoles as well as 3H‐indole iodides was developed for the first time. Using a new imine reductase identified from Paenibacillus lactis (PlSIR), various chiral indolines were facilely synthesized in good yields and excellent enantiopurities (up to >99% ee) under mild reaction conditions.
中文翻译:

利用乳酸芽孢杆菌的亚胺还原酶高效合成手性吲哚啉
为高效不对称还原3的酶促过程ħ以及3个-indoles ħ -吲哚碘化物被首次开发的。使用从乳酸杆菌(Paenibacillus lactis)(PISIR)中鉴定出的一种新的亚胺还原酶,可以在温和的反应条件下以高收率和优异的对映体纯度(最高> 99%ee)轻松合成各种手性二氢吲哚。
更新日期:2015-05-12
中文翻译:

利用乳酸芽孢杆菌的亚胺还原酶高效合成手性吲哚啉
为高效不对称还原3的酶促过程ħ以及3个-indoles ħ -吲哚碘化物被首次开发的。使用从乳酸杆菌(Paenibacillus lactis)(PISIR)中鉴定出的一种新的亚胺还原酶,可以在温和的反应条件下以高收率和优异的对映体纯度(最高> 99%ee)轻松合成各种手性二氢吲哚。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号