当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Efficiency of Primary β-Amino Alcohols and Their Derivatives in Organocatalysis
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2016-04-26 , DOI: 10.1002/ejoc.201600164
Ummareddy Venkata Subba Reddy 1 , Madhu Chennapuram 1 , Chigusa Seki 1 , Eunsang Kwon 2 , Yuko Okuyama 3 , Hiroto Nakano 1
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2016-04-26 , DOI: 10.1002/ejoc.201600164
Ummareddy Venkata Subba Reddy 1 , Madhu Chennapuram 1 , Chigusa Seki 1 , Eunsang Kwon 2 , Yuko Okuyama 3 , Hiroto Nakano 1
Affiliation
|
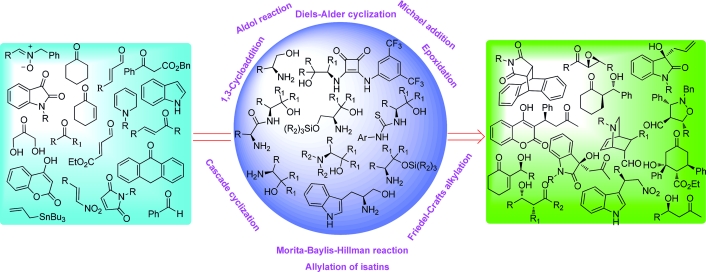
Chiral primary β-amino alcohols, constuting adjacently positioned Bronsted base and Bronsted acid sites, are emerging as very valuable bifunctional organocatalysts in a wide array of asymmetric organic transformations. Primary β-amino alcohols represent inexpensive alternatives to other primary amino organocatalysts such as chiral diamines and cinchona-alkaloid-derived primary amines, being easy to synthesize and air-stable and offering the potential for introduction of different functional groups and also for alteration of steric sites. Here we reveal the catalytic use of simple primary β-amino alcohols and their derivatives as organocatalysts in Diels–Alder cycloaddition, aldol condensation, Michael addition, 1,3-dipolar cycloaddition, the Morita–Baylis–Hillman reaction, cascade cyclization, allylation of isatins, Friedel–Crafts alkylation and epoxidation of olefins.
中文翻译:

β-氨基伯醇及其衍生物在有机催化中的催化效率
手性伯 β-氨基醇构成相邻的布朗斯台德碱位和布朗斯台德酸位点,正在成为各种不对称有机转化中非常有价值的双功能有机催化剂。β-氨基伯醇是其他伯氨基有机催化剂(如手性二胺和金鸡纳生物碱衍生的伯胺)的廉价替代品,易于合成和空气稳定,具有引入不同官能团和改变空间位阻的潜力网站。在这里,我们揭示了简单的 β-氨基伯醇及其衍生物在 Diels-Alder 环加成、羟醛缩合、迈克尔加成、1,3-偶极环加成、Morita-Baylis-Hillman 反应、级联环化、烯丙基化中的催化用途靛红,
更新日期:2016-04-26
中文翻译:

β-氨基伯醇及其衍生物在有机催化中的催化效率
手性伯 β-氨基醇构成相邻的布朗斯台德碱位和布朗斯台德酸位点,正在成为各种不对称有机转化中非常有价值的双功能有机催化剂。β-氨基伯醇是其他伯氨基有机催化剂(如手性二胺和金鸡纳生物碱衍生的伯胺)的廉价替代品,易于合成和空气稳定,具有引入不同官能团和改变空间位阻的潜力网站。在这里,我们揭示了简单的 β-氨基伯醇及其衍生物在 Diels-Alder 环加成、羟醛缩合、迈克尔加成、1,3-偶极环加成、Morita-Baylis-Hillman 反应、级联环化、烯丙基化中的催化用途靛红,































 京公网安备 11010802027423号
京公网安备 11010802027423号