当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Transition State Models for Understanding the Origin of Chiral Induction in Asymmetric Catalysis
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2016-04-21 00:00:00 , DOI: 10.1021/acs.accounts.6b00053
Raghavan B. Sunoj 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2016-04-21 00:00:00 , DOI: 10.1021/acs.accounts.6b00053
Raghavan B. Sunoj 1
Affiliation
|
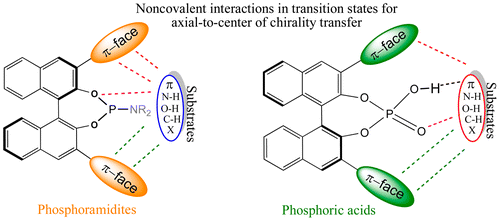
In asymmetric catalysis, a chiral catalyst bearing chiral center(s) is employed to impart chirality to developing stereogenic center(s). A rich and diverse set of chiral catalysts is now available in the repertoire of synthetic organic chemistry. The most recent trends point to the emergence of axially chiral catalysts based on binaphthyl motifs, in particular, BINOL-derived phosphoric acids and phosphoramidites. More fascinating ideas took shape in the form of cooperative multicatalysis wherein organo- and transition-metal catalysts are made to work in concert. At the heart of all such manifestations of asymmetric catalysis, classical or contemporary, is the stereodetermining transition state, which holds a perennial control over the stereochemical outcome of the catalytic process. Delving one step deeper, one would find that the origin of the stereoselectivity is delicately dependent on the relative stabilization of one transition state, responsible for the formation of the predominant stereoisomer, over the other transition state for the minor stereoisomer. The most frequently used working hypothesis to rationalize the experimentally observed stereoselectivity places an undue emphasis on steric factors and tends to regard the same as the origin of facial discrimination between the prochiral faces of the reacting partners. In light of the increasing number of asymmetric catalysts that rely on hydrogen bonding as well as other weak non-covalent interactions, it is important to take cognizance of the involvement of such interactions in the sterocontrolling transition states. Modern density functional theories offer a pragmatic and effective way to capture non-covalent interactions in transition states. Aided by the availability of such improved computational tools, it is quite timely that the molecular origin of stereoselectivity is subjected to more intelligible analysis.
中文翻译:

过渡态模型,用于理解不对称催化中手性诱导的起源
在不对称催化中,带有手性中心的手性催化剂被用于赋予手性以形成立体中心。现在,在合成有机化学中可以使用多种多样的手性催化剂。最近的趋势指出了基于联萘基的轴向手性催化剂的出现,特别是BINOL衍生的磷酸和亚磷酰胺。更有趣的想法以协同多催化的形式形成,其中有机金属和过渡金属催化剂可以协同工作。古典或现代不对称催化的所有这些表现的核心是立体确定的过渡态,该状态长期控制着催化过程的立体化学结果。再深入一步,一个人会发现,立体选择性的起源微妙地取决于一个过渡态的相对稳定,该过渡态负责形成主要的立体异构体,而相对于次要立体异构体的另一个过渡态。使实验观察到的立体选择性合理化的最常用的工作假设过分强调了空间因素,并倾向于将其视为与反应对象前手面之间的面部区别的起因相同。鉴于依赖于氢键以及其他弱的非共价相互作用的不对称催化剂的数量不断增加,重要的是要认识到这种相互作用在立体控制的过渡态中的参与。现代密度泛函理论提供了一种实用且有效的方法来捕获过渡态中的非共价相互作用。借助于这种改进的计算工具的可用性,对立体选择性的分子起源进行更清晰的分析是非常及时的。
更新日期:2016-04-21
中文翻译:

过渡态模型,用于理解不对称催化中手性诱导的起源
在不对称催化中,带有手性中心的手性催化剂被用于赋予手性以形成立体中心。现在,在合成有机化学中可以使用多种多样的手性催化剂。最近的趋势指出了基于联萘基的轴向手性催化剂的出现,特别是BINOL衍生的磷酸和亚磷酰胺。更有趣的想法以协同多催化的形式形成,其中有机金属和过渡金属催化剂可以协同工作。古典或现代不对称催化的所有这些表现的核心是立体确定的过渡态,该状态长期控制着催化过程的立体化学结果。再深入一步,一个人会发现,立体选择性的起源微妙地取决于一个过渡态的相对稳定,该过渡态负责形成主要的立体异构体,而相对于次要立体异构体的另一个过渡态。使实验观察到的立体选择性合理化的最常用的工作假设过分强调了空间因素,并倾向于将其视为与反应对象前手面之间的面部区别的起因相同。鉴于依赖于氢键以及其他弱的非共价相互作用的不对称催化剂的数量不断增加,重要的是要认识到这种相互作用在立体控制的过渡态中的参与。现代密度泛函理论提供了一种实用且有效的方法来捕获过渡态中的非共价相互作用。借助于这种改进的计算工具的可用性,对立体选择性的分子起源进行更清晰的分析是非常及时的。







































 京公网安备 11010802027423号
京公网安备 11010802027423号