当前位置:
X-MOL 学术
›
Eur. J. Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Flexibility of P2O7 Dimers in Soft Structures: M2CdP2O7 (M = Rb, Cs)
European Journal of Inorganic Chemistry ( IF 2.2 ) Pub Date : 2016-04-20 , DOI: 10.1002/ejic.201600086
Xiaoyu Dong 1, 2 , Yunjing Shi 1, 2 , Mingjie Zhang 2 , Zhaohui Chen 1, 3 , Qun Jing 1, 4 , Yun Yang 1 , Shilie Pan 1 , Zhihua Yang 1 , Hongyi Li 1
European Journal of Inorganic Chemistry ( IF 2.2 ) Pub Date : 2016-04-20 , DOI: 10.1002/ejic.201600086
Xiaoyu Dong 1, 2 , Yunjing Shi 1, 2 , Mingjie Zhang 2 , Zhaohui Chen 1, 3 , Qun Jing 1, 4 , Yun Yang 1 , Shilie Pan 1 , Zhihua Yang 1 , Hongyi Li 1
Affiliation
|
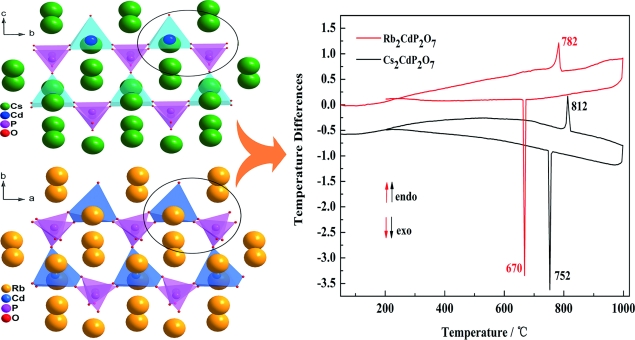
Two new congruent-melting alkali metal diphosphates, Rb2CdP2O7 and Cs2CdP2O7, were synthesized by conventional solid-state reactions. Single-crystal X-ray structural analyses showed that M2CdP2O7 (M = Rb, Cs) feature two-dimensional [CdP2O7](2-) layers that are composed of CdO5 pyramids and P2O7 dimers. The Rb+ and Cs+ cations ions fill the interlayers. The flexible P2O7 dimers could be bent and distorted if the Cs+ ion was substituted by the Rb+ ion, which led to crystallization of the Rb2CdP2O7 compound in the lower symmetry monoclinic space group P2(1)/c (No. 14) and crystallization of the Cs2CdP2O7 compound in the high-symmetry orthorhombic space group Pnma (No. 53). Thermal analyses showed that the two compounds melt congruently and that the melting point of Rb2CdP2O7 compound is lower than that of the Cs2CdP2O7 compound owing to distortion of the P2O7 dimers. IR spectroscopy and UV/Vis-near-IR diffuse reflectance spectroscopy were performed on the reported compounds.
中文翻译:

P2O7 二聚体在软结构中的灵活性:M2CdP2O7 (M = Rb, Cs)
通过常规固态反应合成了两种新的同质熔融碱金属二磷酸盐 Rb2CdP2O7 和 Cs2CdP2O7。单晶 X 射线结构分析表明 M2CdP2O7 (M = Rb, Cs) 具有二维 [CdP2O7](2-) 层,由 CdO5 金字塔和 P2O7 二聚体组成。Rb+ 和 Cs+ 阳离子填充夹层。如果 Cs+ 离子被 Rb+ 离子取代,柔性 P2O7 二聚体可能会弯曲和扭曲,这导致 Rb2CdP2O7 化合物在下对称单斜空间群 P2(1)/c (No. 14) 中结晶和结晶高对称正交空间群 Pnma 中的 Cs2CdP2O7 化合物(第 53 号)。热分析表明,这两种化合物熔融一致,由于 P2O7 二聚体的变形,Rb2CdP2O7 化合物的熔点低于 Cs2CdP2O7 化合物的熔点。对报告的化合物进行了红外光谱和紫外/可见近红外漫反射光谱。
更新日期:2016-04-20
中文翻译:

P2O7 二聚体在软结构中的灵活性:M2CdP2O7 (M = Rb, Cs)
通过常规固态反应合成了两种新的同质熔融碱金属二磷酸盐 Rb2CdP2O7 和 Cs2CdP2O7。单晶 X 射线结构分析表明 M2CdP2O7 (M = Rb, Cs) 具有二维 [CdP2O7](2-) 层,由 CdO5 金字塔和 P2O7 二聚体组成。Rb+ 和 Cs+ 阳离子填充夹层。如果 Cs+ 离子被 Rb+ 离子取代,柔性 P2O7 二聚体可能会弯曲和扭曲,这导致 Rb2CdP2O7 化合物在下对称单斜空间群 P2(1)/c (No. 14) 中结晶和结晶高对称正交空间群 Pnma 中的 Cs2CdP2O7 化合物(第 53 号)。热分析表明,这两种化合物熔融一致,由于 P2O7 二聚体的变形,Rb2CdP2O7 化合物的熔点低于 Cs2CdP2O7 化合物的熔点。对报告的化合物进行了红外光谱和紫外/可见近红外漫反射光谱。

































 京公网安备 11010802027423号
京公网安备 11010802027423号