当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Orbital theory for diastereoselectivity in electrophilic addition
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2016-04-19 06:17:20 Yuji Naruse, Yousuke Hasegawa, Kurumi Ikemoto
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2016-04-19 06:17:20 Yuji Naruse, Yousuke Hasegawa, Kurumi Ikemoto
|
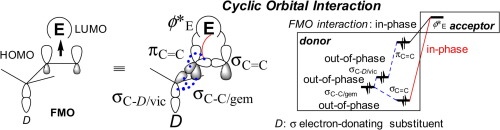
Electrophilic attack to a double bond is often observed anti to the most electron-donating σ-bond at the α-position (hereafter, we refer to this as the extended anomeric effect). This preference is believed to result from the antiperiplanar effect between the bond that is formed between the double bond and the electrophilic reagent, and the donating vicinal σ-bond which is located on the substituent at the α-position. From an orbital viewpoint, however, it is still unclear why the approach of the electrophile anti to the substituent results in stabilization or why the frontier molecular orbital (FMO) deforms, expanding toward the reagent with this antiperiplanar interaction. We demonstrate here that cyclic orbital interaction including geminal bond participation plays an important role in the diastereoselectivity in electrophilic addition. We examined our idea using the electrophilic addition of chlorine to 3-substituted propenes as a model reaction. Our bond model approach should contribute to a better understanding of orbital mixing in FMO.
中文翻译:

亲电加成反应中非对映选择性的轨道理论
经常观察到对双键的亲电子攻击是在α位上与最大电子给体的σ键相反的(此后,我们将其称为扩展的异头效应)。认为该优选是由于在双键和亲电试剂之间形成的键与位于α-取代基上的供体邻位σ-键之间的反周平面作用引起的。然而,从轨道的观点来看,尚不清楚为什么亲电试剂抗取代基的方法会导致稳定化,或者为什么前沿分子轨道(FMO)会变形,并通过这种反周平面的相互作用向试剂方向扩展。我们在这里证明,包括双键参与的循环轨道相互作用在亲电加成的非对映选择性中起重要作用。我们通过将氯亲电加成至3个取代的丙烯中作为模型反应来检验了我们的想法。我们的债券模型方法应有助于更好地理解FMO中的轨道混合。
更新日期:2016-04-19
中文翻译:

亲电加成反应中非对映选择性的轨道理论
经常观察到对双键的亲电子攻击是在α位上与最大电子给体的σ键相反的(此后,我们将其称为扩展的异头效应)。认为该优选是由于在双键和亲电试剂之间形成的键与位于α-取代基上的供体邻位σ-键之间的反周平面作用引起的。然而,从轨道的观点来看,尚不清楚为什么亲电试剂抗取代基的方法会导致稳定化,或者为什么前沿分子轨道(FMO)会变形,并通过这种反周平面的相互作用向试剂方向扩展。我们在这里证明,包括双键参与的循环轨道相互作用在亲电加成的非对映选择性中起重要作用。我们通过将氯亲电加成至3个取代的丙烯中作为模型反应来检验了我们的想法。我们的债券模型方法应有助于更好地理解FMO中的轨道混合。

































 京公网安备 11010802027423号
京公网安备 11010802027423号