当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A catalyst and additive-free three-component reaction of highly electrophilic azides with cyclic ketones and cycloaliphatic amines. Synthesis of novel N-heteroaryl amidines
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2016-04-09 12:13:22
Ilya Efimov, Nikolai Beliaev, Tetyana Beryozkina, Pavel Slepukhin, Vasiliy Bakulev
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2016-04-09 12:13:22
Ilya Efimov, Nikolai Beliaev, Tetyana Beryozkina, Pavel Slepukhin, Vasiliy Bakulev
|
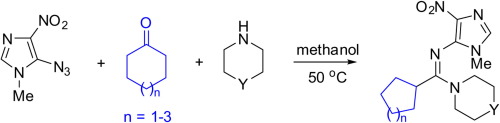
Highly electrophilic 5-azido-1-methyl-4-nitro-1H-imidazole and sulfonyl azides were demonstrated to react with alicyclic amines and cyclic ketones in the absence of any catalyst or additive to afford novel N-(4-nitroimidazol-5-yl)- or N-sulfonylamidines respectively. Based on single crystal X-ray analysis, a revision of the previously reported data of Gao and co-workers on the direction of the reaction of sulfonyl azides with endocyclic enamines was made. The reaction of 2,6-diazidopyridine with an enamine, 4-(cyclohex-1-en-1-yl)morpholine, proceeded with cyclization of the azide moiety onto the pyridine CN bond to form an amidine bearing the tetrazolo[1,5-a]pyridine fragment.
中文翻译:

高度亲电的叠氮化物与环酮和脂环族胺的无催化剂和无添加剂三组分反应。新型N-杂芳基am的合成
高度亲电的5-叠氮基-1-甲基-4-硝基-1H-咪唑和磺酰基叠氮化物在不存在任何催化剂或添加剂的情况下与脂环族胺和环酮反应生成新的N-(4-硝基咪唑-5- yl)-或N-磺酰胺基。基于单晶X射线分析,对Gao及其同事先前报道的有关磺酰叠氮化物与环内烯胺反应方向的数据进行了修订。2,6-二叠氮基吡啶与烯胺4-(环己-1-烯-1-基)吗啉的反应,是将叠氮化物部分环化到吡啶CN键上,形成带有四唑[1,5]的idine -a]吡啶片段。
更新日期:2016-04-10
中文翻译:

高度亲电的叠氮化物与环酮和脂环族胺的无催化剂和无添加剂三组分反应。新型N-杂芳基am的合成
高度亲电的5-叠氮基-1-甲基-4-硝基-1H-咪唑和磺酰基叠氮化物在不存在任何催化剂或添加剂的情况下与脂环族胺和环酮反应生成新的N-(4-硝基咪唑-5- yl)-或N-磺酰胺基。基于单晶X射线分析,对Gao及其同事先前报道的有关磺酰叠氮化物与环内烯胺反应方向的数据进行了修订。2,6-二叠氮基吡啶与烯胺4-(环己-1-烯-1-基)吗啉的反应,是将叠氮化物部分环化到吡啶CN键上,形成带有四唑[1,5]的idine -a]吡啶片段。




































 京公网安备 11010802027423号
京公网安备 11010802027423号