CJC | Breaking Report-中科大顾振华团队:可逐步官能化的2,2'-二溴-6,6'-二碘-1,1'-联苯的制备和应用
本文来源于Chinese Journal of Chemistry,欢迎浏览!
Atropisomerism | Asymmetric catalysis | Ring-opening reaction | Chirality | Copper
可逐步官能化的2,2'-二溴-6,6'-二碘-1,1'-联苯的制备和应用阻旋异构体分子在不对称催化、超分子、材料领域有诸多应用,同时天然产物、药物分子等也常常含有阻旋异构结构。邻位大位阻、多取代通常是稳定阻旋手性分子的必要因素,因此温和条件下高效、高对映选择性合成该类化合物极具挑战性。
联芳基阻旋化合物是阻旋手性的重要一类,其广泛的应用导致对其合成的需求不断增加。与其逐个合成不同的功能化阻旋手性分子,选择一种关键的中间体或前体,并基于此进行多样性官能团化是一个有极具吸引力的替代方案。为此,化学家们合成了一些双卤代的联芳基轴手性化合物用于多样性的官能团化转化,目前有部分化合物已经商业化(图一)。
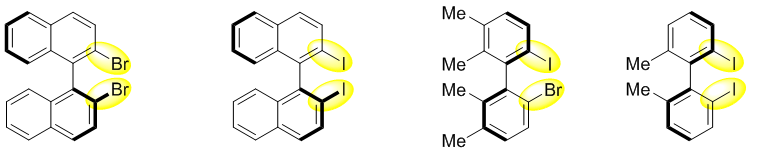
图一、双卤化联芳基轴手性化合物
中国科学技术大学顾振华课题组近年来以环状二芳基碘鎓盐为底物,利用环状化合物的扭转张力实现了温和条件下高对映选择性开环-胺化(芳胺、脂肪胺、三氮唑)、羧酸酯化、硫代羧酸酯化、醚化等,构建了结构多样的碘代联芳基轴手性化合物(Chem 2018, 4, 599; Adv. Synth. Catal. 2018, 360, 3877; ACS Catal. 2019, 9, 9852; Angew. Chem. Int. Ed. 2021, 60, 5788; Angew. Chem. Int. Ed. 2023, 62, e202302749)。最近,该课题组开发了一种铜催化的邻位二溴取代环状二芳基碘鎓与碘化锂的对映选择性开环反应,所得的光学活性产物2,2'-二溴-6,6'-二碘-1,1'-联苯,具有两个C-Br键和两个C-I键(图二)。该化合物可以选择性地逐步官能团化得到一系列官能团化的联苯型轴手性化合物,如芳基碳-碘键与iPrMgCl×LiCl发生碘-镁交换化后可实现羧基化、硼酰化、氧化、烯丙基化和膦化等;所得产物的碳-溴键可与n-BuLi进一步发生溴-锂交换实现再次官能团化。
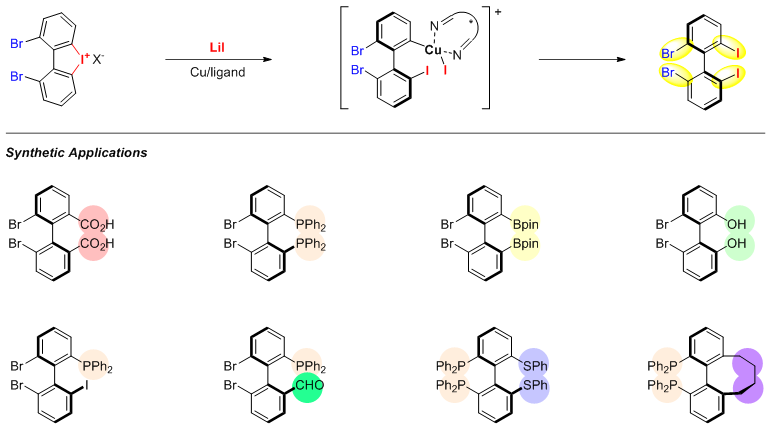
图二、 2,2'-二溴-6,6'-二碘-1,1'-联苯制备及官能团化产物
上述研究结果作为Breaking Report发表于Chin. J. Chem. 2023, 41, 3515—3520. DOI: 10.1002/cjoc.202300438。该项工作得到了国家自然科学基金委、福建省自然科学基金委的资助。点击阅读原文

《中国化学(英文)》(Chinese Journal of Chemistry)创刊于1983年,半月刊,由中国化学会、上海有机化学研究所联合Wiley共同主办。期刊覆盖化学全领域,发表有机化学、无机化学、物理化学、高分子化学、分析化学、材料化学、能源、催化等各学科领域的原始性、创新性成果,期刊历史悠久、审稿流程严格。2022年度影响因子为5.4,JCI指数0.92,5年影响因子4.4,2022年度CiteScore为7.5,SNIP指标为 0. 859。其中科院分区为化学综合2区。期刊先后收录于DOAJ、ESCI、SCIE等数据库。
如果篇首注明了授权来源,任何转载需获得来源方的许可!如果篇首未特别注明出处,本文版权属于 X-MOL ( x-mol.com ), 未经许可,谢绝转载!


































 京公网安备 11010802027423号
京公网安备 11010802027423号