国家留学基金委与巴黎萨克雷大学联合招聘启事

Discipline: Chemistry
Keywords: Organic and Sustainable Chemistry, Homogeneous Catalysis
Doctoral School: “Chemical Sciences” 2MIB-n°571
Host Institute: Institut de Chimie Moléculaire et des Matériaux d'Orsay (ICMMO), UMR 8182, Université Paris Saclay, Orsay, France
https://www.icmmo.u-psud.fr/fr/
Host Group: “Homogeneous Organometallic Catalysis”
https://www.icmmo.universite-paris-saclay.fr/fr/equipes/lcm/homogeneous-organometallic-catalysis/
Group from Laboratory of Molecular Catalysis
https://www.icmmo.universite-paris-saclay.fr/fr/equipes/lcm/homogeneous-organometallic-catalysis/
PhD Supervisor: Dr R. Gil (Associate Prof.), richard.gil@universite-paris-saclay.fr
PhD proposal
Diarylmethylamines and Triarylmethanes synthesized from Friedel-Crafts Additions catalysed by Rare-Earth Complexes
The Friedel-Crafts reaction, one of the oldest carbon-carbon bond forming processes, is still an attractive method to introduce substituents on aromatic rings. These transformations involving an aromatic compound rich in electrons with an electrophilic substrate often requires the use of a very active Lewis acid such as an electro-deficient metal complex. Originally, the catalysts used were iron(III) or aluminum(III) halides. Increasingly, rare earth halides or triflate[1] demonstrate a great efficiency in the Friedel-Crafts reactions. In our group, we have shown that samarium diiodide is an very efficient catalyst in the addition of various aromatic compounds into alkyl 3,3,3-trifluoropyruvates.[2] Because of their high coordination, rare-earths can be complexed to chiral ligands to promote enantioselective processes. Moreover, we have developed an enantioselective Friedel-Crafts alkylation of indole derivatives catalysed by Yb(OTf)3-pyridylalkylamine complexes as chiral Lewis acids.[3] From many years, we are interested by reactions with atom economy catalysed by abundant metals and specially rare-earths.[4]
Firstly, the PhD proposal consists to assess the enatioselectivity of the Friedel-Crafts addition of an aromatic compound onto and aromatic aldimine catalyzed by chiral enantiopure rare-earth complexes. Enantio-enriched diarylmethylamines, important intermediates for the synthesis of products with pharmaceutical properties, are mainly formed in literature from enantioselective hydrogenation or organometallic addition of diaryl ketimines. In a second time, it could be challenging to produce enantio-enriched chiral triarylmethanes from diarylmethylamines and a different second aromatic compound. It should be noted that some triarylmethanes have antitubercular[5] properties and others are employed as precursor of dyes.
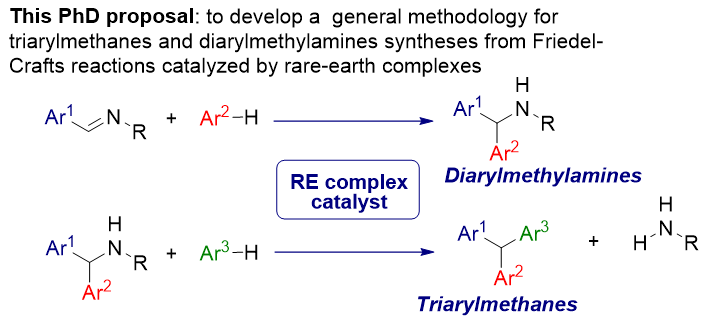
The PhD proposal aims to study enantioselective Friedel-Crafts additions catalysed by chiral rare-earth complexes from aromatic aldimines. In a second step, the formation of chiral triarylmethanes could be obtained from the diarylmethylamines previously formed in the first step. During this PhD work, special attention will be given to the development of complexes of well-defined structure, to their characterizations and to the understanding of the elementary steps including the stereodetermining steps, involved in the catalytic cycles. Considering that the amine RNH2 is not a good leaving group, a stereospecific process with the obtaining of enantio-enriched triarylmethanes could be allowed.
Skills Training in organic synthesis, sustainable chemistry, catalysis and analytical methods will be received by the student and various transferable skills such as communication, writing, time management; project planning and management will be developed.
References:
[1] S. Kobayashi, M. Sugiura, H. Kitagawa, W. W.-L. Lam Chem. Rev. 2002, 102, 2227-2302.
[2] M. Soueidan, J. Collin, R. Gil Tetrahedron Lett. 2006, 47, 5467-5470.
[3] G. Grach, A. Dinut, S. Marque; J. Marrot, R. Gil, D. Prim Org. Biomol. Chem. 2011, 9, 497-503.
[4] V. Rodriguez-Ruiz, R. Carlino, S. Bezzenine-Lafollée, R. Gil, D. Prim, E. Schulz, J. Hannedouche Dalton Trans. 2015, 44, 12029.
[5] M. K. Parai, G. Panda, V. Chaturvedi, Y. K. Manju, S. Sinha Bioorg. Med. Chem. Lett. 2008, 18, 289-292.
如果篇首注明了授权来源,任何转载需获得来源方的许可!如果篇首未特别注明出处,本文版权属于 X-MOL ( x-mol.com ), 未经许可,谢绝转载!
















































 京公网安备 11010802027423号
京公网安备 11010802027423号