RSC主编推荐:有机领域精彩文章快览(免费阅读原文)
英国皇家化学会(RSC)是一个超过175年历史的面向全球化学家的非营利会员制机构,旗下拥有44种期刊,其中很多在化学领域有很高影响力。为了进一步帮助广大读者追踪科技前沿热点,X-MOL团队与英国皇家化学会合作,推出英国皇家化学会期刊主编推荐的精彩文章快览,本期文章属“有机领域”,英文点评来自英国皇家化学会期刊的主编。如果大家对我们的解读有更多的补充和点评,欢迎在文末写评论发表您的高见!
Chemical Science (IF: 9.556)

1. Enantioselective carbene insertion into the N–H bond of benzophenone imine
Chem. Sci., 2019, Advance Article
DOI: 10.1039/C9SC03354H

Researchers from Sichuan University here present the efficient enantioselective insertion of α-diazoesters into the N–H bond of N-sp2-hybridized benzophenone imine using Rh2(esp)2 and chiral quanidine cooperative catalysis, obtaining substituted α-amino esters in high yields with good enantioselectivities.
Open Access(可免费阅读原文)
扫描或长按二维码,识别后直达原文页面,或点此查看原文

2. Nickel-catalyzed hydroalkylation and hydroalkenylation of 1,3-dienes with hydrazones
Chem. Sci., 2019, Advance Article
DOI: 10.1039/C9SC04177J
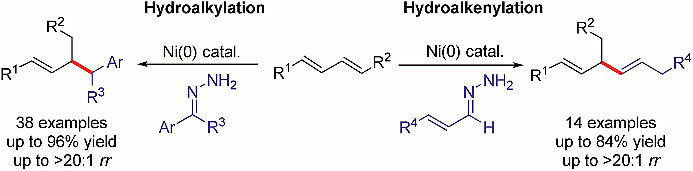
Researchers from Nankai University have reported a protocol for nickel-catalyzed hydroalkylation of dienes with hydrazones, which serve as equivalents of alkyl carbon nucleophiles. In addition, they demonstrate a protocol for hydroalkenylation of dienes with α,β-unsaturated hydrazones, providing a new method for the synthesis of 1,4-dienes.
Open Access(可免费阅读原文)
扫描或长按二维码,识别后直达原文页面,或点此查看原文

Organic Chemistry Frontiers (IF: 5.076)

1. Asymmetric domino 1,6-addition/annulation reaction of 3-cyano-4-alkenyl-2H-chromen-2-ones with isatin-derived MBH carbonates: enantioselective synthesis of 3,3'-cyclopentenylspirooxindoles bearing 2H-chromen-2-ones
Org. Chem. Front., 2019, 6, 3342-3347
DOI: 10.1039/C9QO00890J
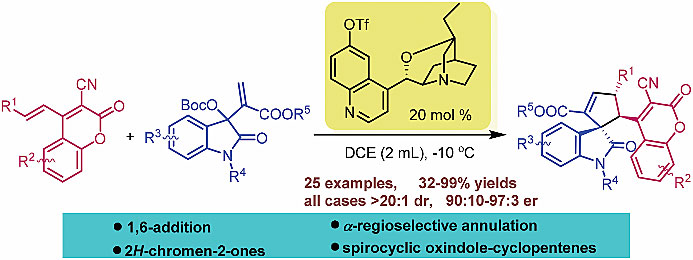
An asymmetric domino 1,6-addition/annulation reaction of 3-cyano-4-alkenyl-2H-chromen-2-ones with isatin-derived MBH carbonates was achieved. With this developed protocol, a series of 3,3′-cyclopentenylspirooxindoles bearing 2H-chromen-2-ones were obtained in moderate to excellent yields with good to excellent diastereo- and enantioselectivities (>20 : 1 dr, up to 97 : 3 er).
限时免费阅读原文,登录后可下载
扫描或长按二维码,识别后直达原文页面,或点此查看原文

2. Highly convergent modular access to poly-carbon substituted cyclopropanes via Cu(I)-catalyzed three-component cyclopropene carboallylation
Org. Chem. Front., 2019, 6, 3387-3391
DOI: 10.1039/C9QO00902G
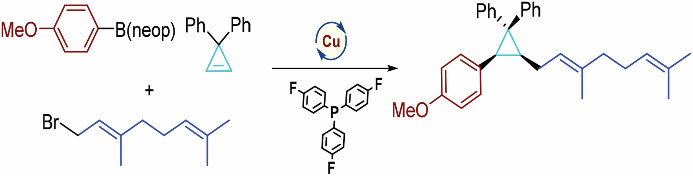
We report herein the first example of conjunctive C–C cross-coupling of cyclopropenes enabled by a Cu-catalyzed three-component reaction of organoboron, cyclopropene and allylic bromide, which features a modular, stereoselective assembly of poly-carbon substituted cyclopropanes. Preliminary studies showed that the reaction can be extended to benzylation and made enantioselective with C2-symmetrical bisphosphine ligands.
限时免费阅读原文,登录后可下载
扫描或长按二维码,识别后直达原文页面,或点此查看原文

如果篇首注明了授权来源,任何转载需获得来源方的许可!如果篇首未特别注明出处,本文版权属于 X-MOL ( x-mol.com ), 未经许可,谢绝转载!































 京公网安备 11010802027423号
京公网安备 11010802027423号