余桂华、赵宇团队Angew:“双氧化还原共熔电解液”助力高性能非水有机液流电池
相比于传统液流电池,非水体系的有机液流电池的工作电压能够克服水分解电压的限制,理论能量密度可以更高。然而在实际应用中,非水有机液流电池面临诸多问题:有机活性分子溶解度低、正负极电解液相互污染、电解液及隔膜离子电导率低等,这些因素都降低了非水有机液流电池的实际操作电流、功率密度和能量密度。如何优化电解液组分,提升非水有机液流电池的性能是研究者亟待解决的问题。
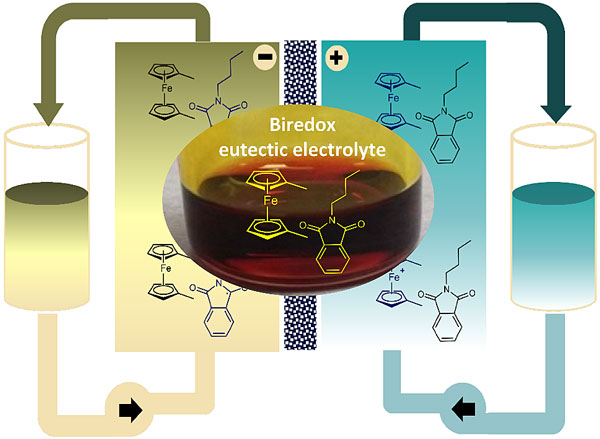
图1. 双氧化还原共熔电解液的构建高性能有机液流电池。图片来源:Angew. Chem. Int. Ed.
近日,美国德克萨斯大学奥斯汀分校的余桂华教授(点击查看介绍)团队与苏州大学的赵宇教授(点击查看介绍)合作,开发了一种被称作“双氧化还原共熔电解液(biredox eutectic electrolyte)”的高浓度有机电解液。直接利用正负极有机活性分子间的分子间相互作用力,在室温下实现高浓度、双极性的共熔电解液。该电解液不仅能够提高活性分子的浓度,而且能够有效缓解正负极电解液的相互污染。他们基于N-丁基-邻苯二甲酰亚胺(BuPh)和1,1-二甲基二茂铁(DMFc)活性分子制备了这种双氧化还原共熔电解液。理论分析发现BuPh和DMFc分子之间形成了强的π-π和Van Der Waals相互作用力。进一步电池测试表明在1.0 M浓度下电池的功率密度可达192 mW cm-2,远优于文献报道的非水有机体系。在大电流60 mA cm-2下完全充放电循环500圈后,电池的容量依然稳定在72%,库伦效率和能量效率分别为93%和51%。在超高浓度下(2 M)双氧化还原共熔电解液依然能够保持稳定的效率和容量输出。
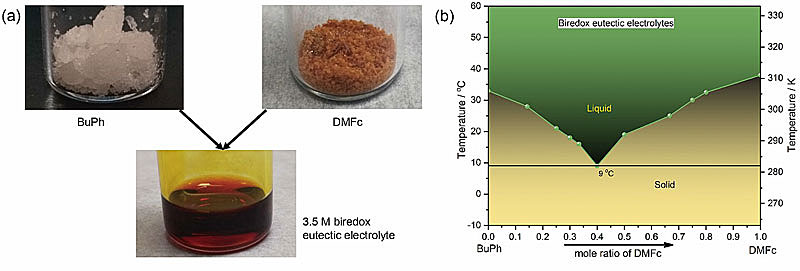
图2. a) 基于BuPh和DMFc制备双氧化还原共熔电解液的策略。b) 双氧化还原共熔电解液的相图。图片来源:Angew. Chem. Int. Ed.
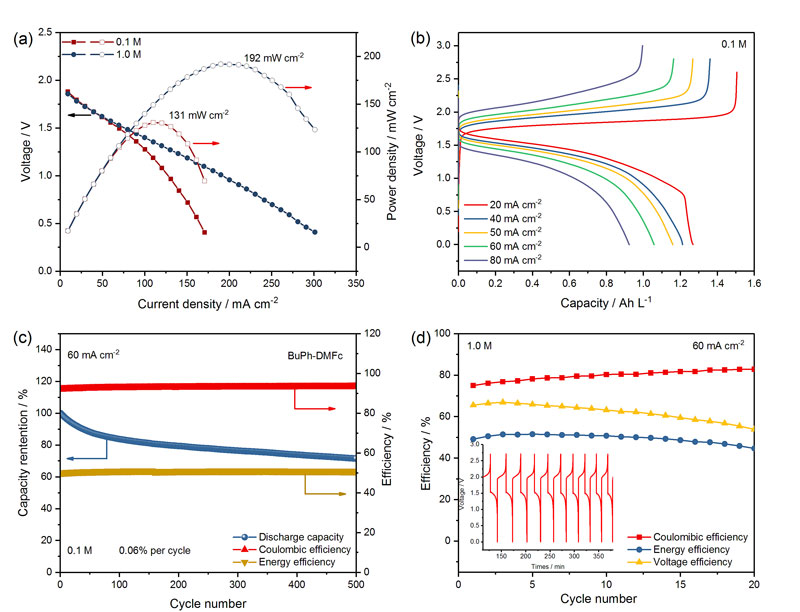
图3. a) 0.1 M和1.0 M电解液的极化曲线。b) 0.1 M电解液不同电流密度下的充放电曲线。c) 0.1 M浓度下电池的循环稳定性和效率值。d) 1.0 M浓度下电池的效率值。图片来源:Angew. Chem. Int. Ed.
这一成果近期发表在Angewandte Chemie International Edition 上。
原文(扫描或长按二维码,识别后直达原文页面,或点此查看原文):

Biredox Eutectic Electrolytes Derived from Organic Redox-Active Molecules: High-Energy Storage Systems
Changkun Zhang, Yumin Qian, Yu Ding, Leyuan Zhang, Xuelin Guo, Yu Zhao, Guihua Yu
Angew. Chem. Int. Ed., 2019, 58, 7045-7050, DOI. 10.1002/anie.201902433
余桂华教授团队简介
余桂华教授团队近些年致力于新型液流电池的研究和设计,综合了化学科学、材料科学和能源科学的跨学科研究,包括通过有机合成对活性材料的物理/化学特性的优化,利用共熔体系独特的优势构建高浓度的电解液,同时结合分子水平的电化学反应机理和反应动力学研究,借助理论的计算分析,发展了一系列新型有机液流电池、仿生液流电池。在液流电池领域的更多重大开创性工作和综述文章可见:
1. A chemistry and material perspective on lithium redox flow batteries towards high-density electrical energy storage. Chem. Soc. Rev., 2015, 44, 7968.
2. Molecular engineering of organic electroactive materials for redox flow batteries. Chem. Soc. Rev., 2018, 47, 69.
3. A high-performance all-metallocene-based, non-aqueous redox flow battery. Energy Environ. Sci., 2017, 10, 491.
4. A reversible Br2/Br redox couple in the aqueous phase as a high-performance catholyte for alkali-ion batteries. Energy Environ. Sci., 2014, 7, 1990.
5. A Low-Cost and High-Energy Hybrid Iron-Aluminum Liquid Battery Achieved by Deep Eutectic Solvents. Joule, 2017, 1, 623.
6. Sustainable Electrical Energy Storage through the Ferrocene/Ferrocenium Redox Reaction in Aprotic Electrolyte. Angew. Chem. Int. Ed., 2014, 53, 11036.
7. A Bio-Inspired, Heavy-Metal-Free, Dual-Electrolyte Liquid Battery towards Sustainable Energy Storage. Angew. Chem. Int. Ed., 2016, 55, 4772.
8. A Sustainable Redox-Flow Battery with an Aluminum-Based, Deep-Eutectic-Solvent Anolyte. Angew. Chem. Int. Ed., 2017, 56, 7454.
9. Exploring Bio-inspired Quinone-Based Organic Redox Flow Batteries: A Combined Experimental and Computational Study. Chem, 2016, 1, 790.
10. Enabling Graphene-Oxide-Based Membranes for Large-Scale Energy Storage by Controlling Hydrophilic Microstructures. Chem, 2018. 4, 1035.
11. Highly Concentrated Phthalimide-Based Anolytes for Organic Redox Flow Batteries with Enhanced Reversibility. Chem, 2018, 4, 2814.
12. A Membrane-Free Ferrocene-Based High-Rate Semiliquid Battery. Nano Lett., 2015, 15, 4108.
13. Insights into Hydrotropic Solubilization for Hybrid Ion Redox Flow Batteries. ACS Energy Lett., 2018, 3, 2641.
14. Eutectic Electrolytes for High-Energy-Density Redox Flow Batteries. ACS Energy Lett., 2018, 3, 2875.
15. Progress and Prospects of Next-Generation Redox Flow Batteries. Energy Storage Mater., 2018, 15, 324.
如果篇首注明了授权来源,任何转载需获得来源方的许可!如果篇首未特别注明出处,本文版权属于 X-MOL ( x-mol.com ), 未经许可,谢绝转载!
































 京公网安备 11010802027423号
京公网安备 11010802027423号