当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oxygen-Promoted Methane Activation on Copper
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2017-11-16 00:00:00 , DOI: 10.1021/acs.jpcb.7b06956 Tianchao Niu 1, 2 , Zhao Jiang 3 , Yaguang Zhu 4 , Guangwen Zhou 4 , Matthijs A. van Spronsen 5 , Samuel A. Tenney 1 , J. Anibal Boscoboinik 1 , Dario Stacchiola 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2017-11-16 00:00:00 , DOI: 10.1021/acs.jpcb.7b06956 Tianchao Niu 1, 2 , Zhao Jiang 3 , Yaguang Zhu 4 , Guangwen Zhou 4 , Matthijs A. van Spronsen 5 , Samuel A. Tenney 1 , J. Anibal Boscoboinik 1 , Dario Stacchiola 1
Affiliation
|
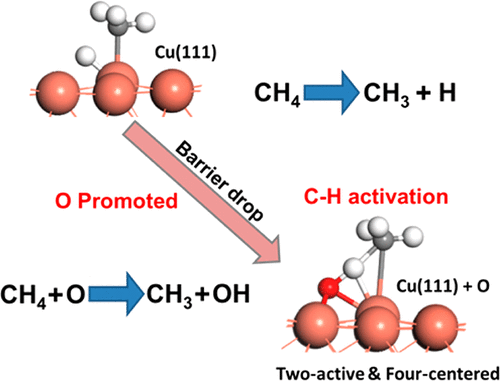
The role of oxygen in the activation of C–H bonds in methane on clean and oxygen-precovered Cu(111) and Cu2O(111) surfaces was studied with combined in situ near-ambient-pressure scanning tunneling microscopy and X-ray photoelectron spectroscopy. Activation of methane at 300 K and “moderate pressures” was only observed on oxygen-precovered Cu(111) surfaces. Density functional theory calculations reveal that the lowest activation energy barrier of C–H on Cu(111) in the presence of chemisorbed oxygen is related to a two-active-site, four-centered mechanism, which stabilizes the required transition-state intermediate by dipole–dipole attraction of O–H and Cu–CH3 species. The C–H bond activation barriers on Cu2O(111) surfaces are large due to the weak stabilization of H and CH3 fragments.
中文翻译:

氧促进铜上甲烷的活化
通过结合原位近环境压力扫描隧道显微镜和X射线研究了氧气在清洁的和氧气覆盖的Cu(111)和Cu 2 O(111)表面上甲烷中C–H键的活化中的作用。光电子能谱。仅在氧覆盖的Cu(111)表面上观察到300 K和“中等压力”下的甲烷活化。密度泛函理论计算表明,在化学吸附的氧存在下,C–H在Cu(111)上的最低活化能垒与两个活性位点,四个中心的机理有关,该机理稳定了所需的过渡态中间体。 O–H和Cu–CH 3物种的偶极子-偶极子吸引力。Cu 2上的C–H键激活势垒由于H和CH 3片段的稳定性较弱,O(111)表面较大。
更新日期:2017-11-17
中文翻译:

氧促进铜上甲烷的活化
通过结合原位近环境压力扫描隧道显微镜和X射线研究了氧气在清洁的和氧气覆盖的Cu(111)和Cu 2 O(111)表面上甲烷中C–H键的活化中的作用。光电子能谱。仅在氧覆盖的Cu(111)表面上观察到300 K和“中等压力”下的甲烷活化。密度泛函理论计算表明,在化学吸附的氧存在下,C–H在Cu(111)上的最低活化能垒与两个活性位点,四个中心的机理有关,该机理稳定了所需的过渡态中间体。 O–H和Cu–CH 3物种的偶极子-偶极子吸引力。Cu 2上的C–H键激活势垒由于H和CH 3片段的稳定性较弱,O(111)表面较大。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号