Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
MCT1 and MCT4 Expression and Lactate Flux Activity Increase During White and Brown Adipogenesis and Impact Adipocyte Metabolism.
Scientific Reports ( IF 3.8 ) Pub Date : 2017-Oct-12 , DOI: 10.1038/s41598-017-13298-z
Charlotte Petersen , Mette D. Nielsen , Elise S. Andersen , Astrid L. Basse , Marie S. Isidor , Lasse K. Markussen , Birgitte M. Viuff , Ian H. Lambert , Jacob B. Hansen , Stine F. Pedersen
Scientific Reports ( IF 3.8 ) Pub Date : 2017-Oct-12 , DOI: 10.1038/s41598-017-13298-z
Charlotte Petersen , Mette D. Nielsen , Elise S. Andersen , Astrid L. Basse , Marie S. Isidor , Lasse K. Markussen , Birgitte M. Viuff , Ian H. Lambert , Jacob B. Hansen , Stine F. Pedersen
|
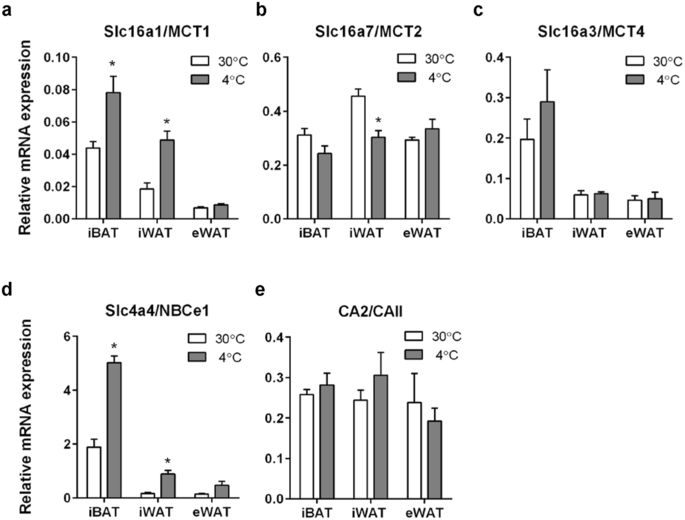
Adipose tissue takes up glucose and releases lactate, thereby contributing significantly to systemic glucose and lactate homeostasis. This implies the necessity of upregulation of net acid and lactate flux capacity during adipocyte differentiation and function. However, the regulation of lactate- and acid/base transporters in adipocytes is poorly understood. Here, we tested the hypothesis that adipocyte thermogenesis, browning and differentiation are associated with an upregulation of plasma membrane lactate and acid/base transport capacity that in turn is important for adipocyte metabolism. The mRNA and protein levels of the lactate-H+ transporter MCT1 and the Na+,HCO3- cotransporter NBCe1 were upregulated in mouse interscapular brown and inguinal white adipose tissue upon cold induction of thermogenesis and browning. MCT1, MCT4, and NBCe1 were furthermore strongly upregulated at the mRNA and protein level upon differentiation of cultured pre-adipocytes. Adipocyte differentiation was accompanied by increased plasma membrane lactate flux capacity, which was reduced by MCT inhibition and by MCT1 knockdown. Finally, in differentiated brown adipocytes, glycolysis (assessed as ECAR), and after noradrenergic stimulation also oxidative metabolism (OCR), was decreased by MCT inhibition. We suggest that upregulation of MCT1- and MCT4-mediated lactate flux capacity and NBCe1-mediated HCO3-/pH homeostasis are important for the physiological function of mature adipocytes.
中文翻译:

在白色和棕色脂肪形成和影响脂肪细胞代谢过程中,MCT1和MCT4的表达和乳酸通量活性增加。
脂肪组织吸收葡萄糖并释放乳酸,从而显着促进全身性葡萄糖和乳酸的体内稳态。这意味着在脂肪细胞分化和功能过程中必须上调净酸和乳酸通量。然而,人们对脂肪细胞中乳酸和酸/碱转运蛋白的调控知之甚少。在这里,我们测试了以下假设:脂肪细胞的生热,褐变和分化与质膜乳酸和酸/碱转运能力的上调有关,而这反过来对脂肪细胞的代谢很重要。乳酸-H的mRNA和蛋白质水平+转运MCT1和中的Na +,HCO 3 -冷诱导的生热和褐变后,小鼠肩cap间褐色和腹股沟白色脂肪组织中的cotransporter NBCe1上调。在培养的前脂肪细胞分化后,MCT1,MCT4和NBCe1在mRNA和蛋白质水平上也被强烈上调。脂肪细胞分化伴随着质膜乳酸通量增加,而MCT抑制和MCT1敲低则降低了通量。最后,在分化的棕色脂肪细胞中,糖酵解(评估为ECAR),以及在去甲肾上腺素能刺激后,MCT抑制作用还降低了氧化代谢(OCR)。我们建议MCT1-和MCT4介导的乳酸通量能力和NBCe1介导HCO的那上调3 - / pH值平衡是成熟脂肪细胞的生理功能非常重要。
更新日期:2017-10-12
中文翻译:

在白色和棕色脂肪形成和影响脂肪细胞代谢过程中,MCT1和MCT4的表达和乳酸通量活性增加。
脂肪组织吸收葡萄糖并释放乳酸,从而显着促进全身性葡萄糖和乳酸的体内稳态。这意味着在脂肪细胞分化和功能过程中必须上调净酸和乳酸通量。然而,人们对脂肪细胞中乳酸和酸/碱转运蛋白的调控知之甚少。在这里,我们测试了以下假设:脂肪细胞的生热,褐变和分化与质膜乳酸和酸/碱转运能力的上调有关,而这反过来对脂肪细胞的代谢很重要。乳酸-H的mRNA和蛋白质水平+转运MCT1和中的Na +,HCO 3 -冷诱导的生热和褐变后,小鼠肩cap间褐色和腹股沟白色脂肪组织中的cotransporter NBCe1上调。在培养的前脂肪细胞分化后,MCT1,MCT4和NBCe1在mRNA和蛋白质水平上也被强烈上调。脂肪细胞分化伴随着质膜乳酸通量增加,而MCT抑制和MCT1敲低则降低了通量。最后,在分化的棕色脂肪细胞中,糖酵解(评估为ECAR),以及在去甲肾上腺素能刺激后,MCT抑制作用还降低了氧化代谢(OCR)。我们建议MCT1-和MCT4介导的乳酸通量能力和NBCe1介导HCO的那上调3 - / pH值平衡是成熟脂肪细胞的生理功能非常重要。

































 京公网安备 11010802027423号
京公网安备 11010802027423号