当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nickel‐Catalyzed Cyanation of Aryl Chlorides and Triflates Using Butyronitrile: Merging Retro‐hydrocyanation with Cross‐Coupling
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-11-07 , DOI: 10.1002/anie.201707517 Peng Yu 1 , Bill Morandi 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-11-07 , DOI: 10.1002/anie.201707517 Peng Yu 1 , Bill Morandi 1
Affiliation
|
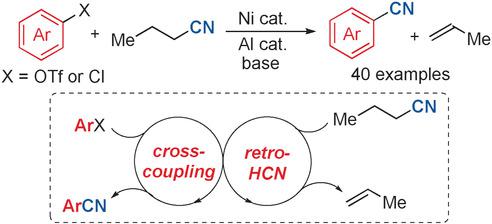
We describe a nickel‐catalyzed cyanation reaction of aryl (pseudo)halides that employs butyronitrile as a cyanating reagent instead of highly toxic cyanide salts. A dual catalytic cycle merging retro‐hydrocyanation and cross‐coupling enables the conversion of a broad array of aryl chlorides and aryl/vinyl triflates into their corresponding nitriles. This new reaction provides a strategically distinct approach to the safe preparation of aryl cyanides, which are essential compounds in agrochemistry and medicinal chemistry.
中文翻译:

丁腈对镍催化的芳基氯化物和三氟甲磺酸酯的氰化反应:将逆向氢氰化与交叉偶联合并
我们描述了芳基(假)卤化物的镍催化氰化反应,该反应采用丁腈作为氰化试剂代替剧毒的氰化物盐。将逆向氢氰化和交叉偶联相结合的双催化循环可将多种芳基氯和芳基/乙烯基三氟甲磺酸转化为相应的腈。该新反应为安全制备芳基氰化物提供了战略上独特的方法,芳基氰化物是农业化学和药物化学中必不可少的化合物。
更新日期:2017-11-07
中文翻译:

丁腈对镍催化的芳基氯化物和三氟甲磺酸酯的氰化反应:将逆向氢氰化与交叉偶联合并
我们描述了芳基(假)卤化物的镍催化氰化反应,该反应采用丁腈作为氰化试剂代替剧毒的氰化物盐。将逆向氢氰化和交叉偶联相结合的双催化循环可将多种芳基氯和芳基/乙烯基三氟甲磺酸转化为相应的腈。该新反应为安全制备芳基氰化物提供了战略上独特的方法,芳基氰化物是农业化学和药物化学中必不可少的化合物。















































 京公网安备 11010802027423号
京公网安备 11010802027423号