Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rev1 contributes to proper mitochondrial function via the PARP-NAD+-SIRT1-PGC1α axis.
Scientific Reports ( IF 3.8 ) Pub Date : 2017-Oct-02 , DOI: 10.1038/s41598-017-12662-3 Nima Borhan Fakouri 1 , Jon Ambæk Durhuus 1 , Christine Elisabeth Regnell 2, 3 , Maria Angleys 1 , Claus Desler 1 , Md Mahdi Hasan-Olive 2 , Ana Martín-Pardillos 4 , Anastasia Tsaalbi-Shtylik 4 , Kirsten Thomsen 3 , Martin Lauritzen 3, 5 , Vilhelm A Bohr 1, 6 , Niels de Wind 4 , Linda Hildegard Bergersen 2, 3 , Lene Juel Rasmussen 1
Scientific Reports ( IF 3.8 ) Pub Date : 2017-Oct-02 , DOI: 10.1038/s41598-017-12662-3 Nima Borhan Fakouri 1 , Jon Ambæk Durhuus 1 , Christine Elisabeth Regnell 2, 3 , Maria Angleys 1 , Claus Desler 1 , Md Mahdi Hasan-Olive 2 , Ana Martín-Pardillos 4 , Anastasia Tsaalbi-Shtylik 4 , Kirsten Thomsen 3 , Martin Lauritzen 3, 5 , Vilhelm A Bohr 1, 6 , Niels de Wind 4 , Linda Hildegard Bergersen 2, 3 , Lene Juel Rasmussen 1
Affiliation
|
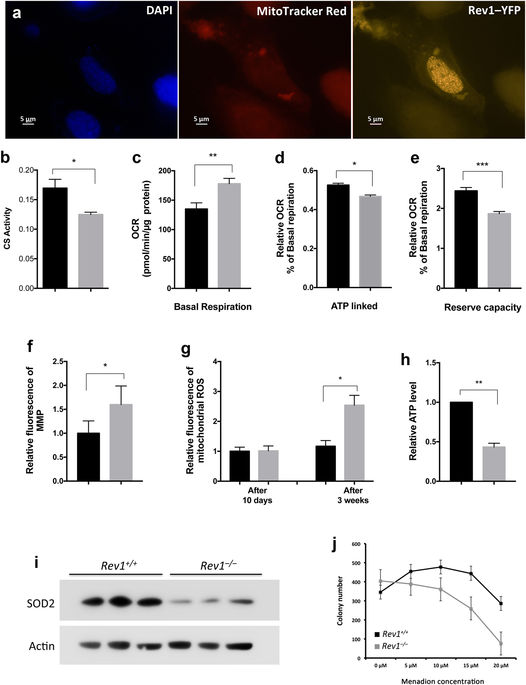
Nucleic acids, which constitute the genetic material of all organisms, are continuously exposed to endogenous and exogenous damaging agents, representing a significant challenge to genome stability and genome integrity over the life of a cell or organism. Unrepaired DNA lesions, such as single- and double-stranded DNA breaks (SSBs and DSBs), and single-stranded gaps can block progression of the DNA replication fork, causing replicative stress and/or cell cycle arrest. However, translesion synthesis (TLS) DNA polymerases, such as Rev1, have the ability to bypass some DNA lesions, which can circumvent the process leading to replication fork arrest and minimize replicative stress. Here, we show that Rev1-deficiency in mouse embryo fibroblasts or mouse liver tissue is associated with replicative stress and mitochondrial dysfunction. In addition, Rev1-deficiency is associated with high poly(ADP) ribose polymerase 1 (PARP1) activity, low endogenous NAD+, low expression of SIRT1 and PGC1α and low adenosine monophosphate (AMP)-activated kinase (AMPK) activity. We conclude that replication stress via Rev1-deficiency contributes to metabolic stress caused by compromized mitochondrial function via the PARP-NAD+-SIRT1-PGC1α axis.
中文翻译:

Rev1 通过 PARP-NAD+-SIRT1-PGC1α 轴促进线粒体的正常功能。
构成所有生物体遗传物质的核酸不断暴露于内源性和外源性损伤剂,这对细胞或生物体生命周期中的基因组稳定性和基因组完整性构成了重大挑战。未修复的 DNA 损伤,例如单链和双链 DNA 断裂(SSB 和 DSB)以及单链缺口,可以阻止 DNA 复制叉的进展,导致复制应激和/或细胞周期停滞。然而,跨损伤合成 (TLS) DNA 聚合酶,例如 Rev1,能够绕过一些 DNA 损伤,从而绕过导致复制叉停滞的过程,并最大限度地减少复制应激。在这里,我们发现小鼠胚胎成纤维细胞或小鼠肝组织中的 Rev1 缺陷与复制应激和线粒体功能障碍有关。此外,Rev1 缺陷与高聚 (ADP) 核糖聚合酶 1 (PARP1) 活性、低内源性 NAD +、SIRT1 和 PGC1α 低表达以及低腺苷单磷酸 (AMP) 激活激酶 (AMPK) 活性相关。我们得出的结论是,Rev1 缺陷导致的复制应激会导致线粒体功能受损(通过 PARP-NAD + -SIRT1-PGC1α 轴)引起的代谢应激。
更新日期:2017-10-02
中文翻译:

Rev1 通过 PARP-NAD+-SIRT1-PGC1α 轴促进线粒体的正常功能。
构成所有生物体遗传物质的核酸不断暴露于内源性和外源性损伤剂,这对细胞或生物体生命周期中的基因组稳定性和基因组完整性构成了重大挑战。未修复的 DNA 损伤,例如单链和双链 DNA 断裂(SSB 和 DSB)以及单链缺口,可以阻止 DNA 复制叉的进展,导致复制应激和/或细胞周期停滞。然而,跨损伤合成 (TLS) DNA 聚合酶,例如 Rev1,能够绕过一些 DNA 损伤,从而绕过导致复制叉停滞的过程,并最大限度地减少复制应激。在这里,我们发现小鼠胚胎成纤维细胞或小鼠肝组织中的 Rev1 缺陷与复制应激和线粒体功能障碍有关。此外,Rev1 缺陷与高聚 (ADP) 核糖聚合酶 1 (PARP1) 活性、低内源性 NAD +、SIRT1 和 PGC1α 低表达以及低腺苷单磷酸 (AMP) 激活激酶 (AMPK) 活性相关。我们得出的结论是,Rev1 缺陷导致的复制应激会导致线粒体功能受损(通过 PARP-NAD + -SIRT1-PGC1α 轴)引起的代谢应激。































 京公网安备 11010802027423号
京公网安备 11010802027423号