Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Armored CAR T cells enhance antitumor efficacy and overcome the tumor microenvironment.
Scientific Reports ( IF 3.8 ) Pub Date : 2017-Sep-05 , DOI: 10.1038/s41598-017-10940-8 Oladapo O Yeku 1 , Terence J Purdon 1 , Mythili Koneru 1 , David Spriggs 1 , Renier J Brentjens 1
Scientific Reports ( IF 3.8 ) Pub Date : 2017-Sep-05 , DOI: 10.1038/s41598-017-10940-8 Oladapo O Yeku 1 , Terence J Purdon 1 , Mythili Koneru 1 , David Spriggs 1 , Renier J Brentjens 1
Affiliation
|
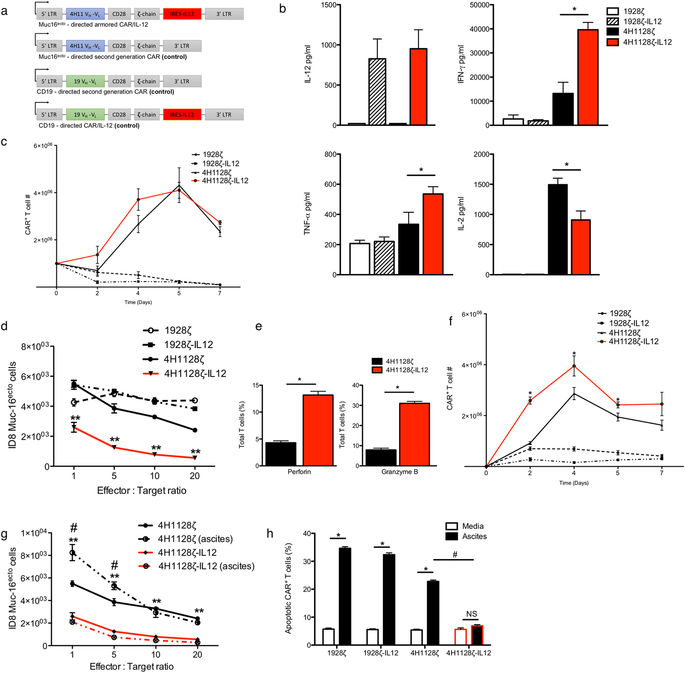
Chimeric antigen receptor (CAR) T cell therapy has shown limited efficacy for the management of solid tumor malignancies. In ovarian cancer, this is in part due to an immunosuppressive cytokine and cellular tumor microenvironment which suppresses adoptively transferred T cells. We engineered an armored CAR T cell capable of constitutive secretion of IL-12, and delineate the mechanisms via which these CAR T cells overcome a hostile tumor microenvironment. In this report, we demonstrate enhanced proliferation, decreased apoptosis and increased cytotoxicity in the presence of immunosuppressive ascites. In vivo, we show enhanced expansion and CAR T cell antitumor efficacy, culminating in improvement in survival in a syngeneic model of ovarian peritoneal carcinomatosis. Armored CAR T cells mediated depletion of tumor associated macrophages and resisted endogenous PD-L1-induced inhibition. These findings highlight the role of the inhibitory microenvironment and how CAR T cells can be further engineered to maintain efficacy.
中文翻译:

装甲CAR T细胞增强抗肿瘤功效并克服肿瘤微环境。
嵌合抗原受体 (CAR) T 细胞疗法对于治疗实体瘤恶性肿瘤的疗效有限。在卵巢癌中,这部分是由于免疫抑制细胞因子和细胞肿瘤微环境抑制了过继转移的 T 细胞。我们设计了一种能够组成性分泌 IL-12 的装甲 CAR T 细胞,并描述了这些 CAR T 细胞克服不利肿瘤微环境的机制。在本报告中,我们证明了在免疫抑制性腹水存在的情况下增殖增强、细胞凋亡减少和细胞毒性增加。在体内,我们展示了增强的扩增和 CAR T 细胞抗肿瘤功效,最终改善了卵巢腹膜癌同基因模型的生存率。装甲 CAR T 细胞介导肿瘤相关巨噬细胞的消耗并抵抗内源性 PD-L1 诱导的抑制。这些发现强调了抑制性微环境的作用以及如何进一步改造 CAR T 细胞以保持功效。
更新日期:2017-09-05
中文翻译:

装甲CAR T细胞增强抗肿瘤功效并克服肿瘤微环境。
嵌合抗原受体 (CAR) T 细胞疗法对于治疗实体瘤恶性肿瘤的疗效有限。在卵巢癌中,这部分是由于免疫抑制细胞因子和细胞肿瘤微环境抑制了过继转移的 T 细胞。我们设计了一种能够组成性分泌 IL-12 的装甲 CAR T 细胞,并描述了这些 CAR T 细胞克服不利肿瘤微环境的机制。在本报告中,我们证明了在免疫抑制性腹水存在的情况下增殖增强、细胞凋亡减少和细胞毒性增加。在体内,我们展示了增强的扩增和 CAR T 细胞抗肿瘤功效,最终改善了卵巢腹膜癌同基因模型的生存率。装甲 CAR T 细胞介导肿瘤相关巨噬细胞的消耗并抵抗内源性 PD-L1 诱导的抑制。这些发现强调了抑制性微环境的作用以及如何进一步改造 CAR T 细胞以保持功效。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号