当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric Dual Chiral Catalysis using Iridium Phosphoramidites and Diarylprolinol Silyl Ethers: Insights into Stereodivergence
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-09-01 00:00:00 , DOI: 10.1021/acscatal.7b02776 Bangaru Bhaskararao 1 , Raghavan B. Sunoj 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-09-01 00:00:00 , DOI: 10.1021/acscatal.7b02776 Bangaru Bhaskararao 1 , Raghavan B. Sunoj 1
Affiliation
|
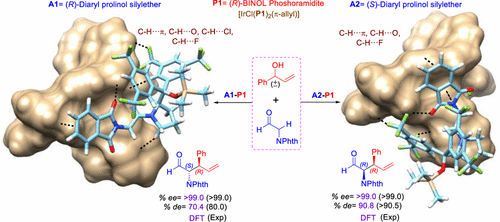
Recent examples of asymmetric dual chiral catalysis (ADCC), where two chiral catalysts are employed under one-pot reaction conditions, have demonstrated how all stereoisomers of a product could be effectively accomplished through changes in the catalyst chirality. Insufficient mechanistic details on the action of two chiral catalysts and molecular insights into the origin of stereodivergence prompted us to undertake a comprehensive density functional theory (B3LYP-D3) investigation on an α-allylation reaction of an aldehyde by using an allyl alcohol, resulting in two new chiral centers in the product. The structural and energetic features of the stereocontrolling transition states helped us delineate how all four product stereoisomers could be accessed by using suitable combinations of chiral iridium phosphoramide and diarylprolinol silyl ether in this ADCC reaction. The covalent activation of the pronucleophile (aldehyde) by the organocatalyst furnishes a chiral enamine, whereas the action of the transition-metal catalyst (chiral Ir phosphoramidite, P) on racemic allyl alcohol gives the Ir-π-allyl phosphoramidite complex [IrCl(P)2(π-allyl)], which serves as the electrophilic partner. The enantioselectivity is directly controlled by the sense of axial chirality of the Ir-bound phosphoramidite ligand, which affects whether an R or S stereocenter would be generated at the β-carbon of the product. The “recognition/interaction” between the two chiral catalysts in the diastereocontrolling C–C bond formation transition states through a series of weak noncovalent interactions (C–H···π, C–H···O, C–H···Cl, C–H···F, and lone pair···π) is identified as playing a pivotal role in influencing the favorable mode of addition of the si or re face of the chiral enamine to Ir-π-allyl phosphoramidite (si-si/re-re) and hence controls the chirality at the α-carbon atom of the developing product.
中文翻译:

铱亚磷酰胺和二芳基脯氨醇甲硅烷基醚的不对称双手性催化:立体发散的见解。
最近的不对称双手性催化(ADCC)的例子表明,如何通过改变催化剂的手性来有效地实现产品的所有立体异构体,其中在一锅法反应条件下使用了两种手性催化剂。关于两种手性催化剂作用的机理细节不足以及对立体发散起源的分子洞察力促使我们进行了全面的密度泛函理论(B3LYP-D3)研究,即利用烯丙醇对醛的α-烯丙基化反应进行了研究,结果发现产品中有两个新的手性中心。立体控制过渡态的结构和能量特征帮助我们描述了如何通过在该ADCC反应中使用手性铱磷酰胺和二芳基脯氨醇甲硅烷基醚的适当组合来访问所有四种产物立体异构体。有机催化剂对亲核试剂(醛)的共价活化提供了手性烯胺,而过渡金属催化剂(手性Ir磷酰胺,外消旋烯丙醇上的P)生成Ir-π-烯丙基亚磷酰胺络合物[IrCl(P)2(π-烯丙基)],它可作为亲电子配合物。对映选择性是直接由Ir结合的亚磷酰胺配体的轴向手性来控制的,这会影响在产物的β-碳上是否会生成R或S立体中心。通过一系列弱的非共价相互作用(C–H···π,C–H··O,C–H·· ·Cl,C–H···F和孤对···π)被认为在影响si或re的有利添加方式中起着关键作用手性烯胺面对Ir-π-烯丙基亚磷酰胺(si-si / re-re),因此控制了显影产物的α-碳原子上的手性。
更新日期:2017-09-04
中文翻译:

铱亚磷酰胺和二芳基脯氨醇甲硅烷基醚的不对称双手性催化:立体发散的见解。
最近的不对称双手性催化(ADCC)的例子表明,如何通过改变催化剂的手性来有效地实现产品的所有立体异构体,其中在一锅法反应条件下使用了两种手性催化剂。关于两种手性催化剂作用的机理细节不足以及对立体发散起源的分子洞察力促使我们进行了全面的密度泛函理论(B3LYP-D3)研究,即利用烯丙醇对醛的α-烯丙基化反应进行了研究,结果发现产品中有两个新的手性中心。立体控制过渡态的结构和能量特征帮助我们描述了如何通过在该ADCC反应中使用手性铱磷酰胺和二芳基脯氨醇甲硅烷基醚的适当组合来访问所有四种产物立体异构体。有机催化剂对亲核试剂(醛)的共价活化提供了手性烯胺,而过渡金属催化剂(手性Ir磷酰胺,外消旋烯丙醇上的P)生成Ir-π-烯丙基亚磷酰胺络合物[IrCl(P)2(π-烯丙基)],它可作为亲电子配合物。对映选择性是直接由Ir结合的亚磷酰胺配体的轴向手性来控制的,这会影响在产物的β-碳上是否会生成R或S立体中心。通过一系列弱的非共价相互作用(C–H···π,C–H··O,C–H·· ·Cl,C–H···F和孤对···π)被认为在影响si或re的有利添加方式中起着关键作用手性烯胺面对Ir-π-烯丙基亚磷酰胺(si-si / re-re),因此控制了显影产物的α-碳原子上的手性。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号