当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Decomposition of Methanol on Mixed CuO–CuWO4 Surfaces
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2017-09-01 00:00:00 , DOI: 10.1021/acs.jpcb.7b06233
M. Blatnik 1 , C. Drechsel 1 , N. Tsud 2 , S. Surnev 1 , F.P. Netzer 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2017-09-01 00:00:00 , DOI: 10.1021/acs.jpcb.7b06233
M. Blatnik 1 , C. Drechsel 1 , N. Tsud 2 , S. Surnev 1 , F.P. Netzer 1
Affiliation
|
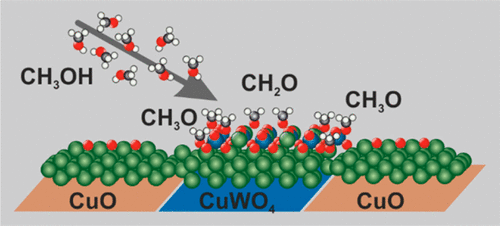
Mixed CuO(2 × 1)–CuWO4 layers on a Cu(110) surface have been prepared by the on-surface reaction of the CuO(2 × 1) surface oxide with adsorbed (WO3)3 clusters. The adsorption and decomposition of methanol on these well-defined CuO–CuWO4 surfaces has been followed by high-resolution X-ray photoelectron spectroscopy (XPS), high-resolution electron energy loss spectroscopy (HREELS), and temperature-programmed desorption (TPD) to assess the molecular surface species and their concentration, while the state of the surface oxide phases before and after methanol decomposition has been characterized by scanning tunneling microscopy (STM), low energy electron diffraction (LEED), and XPS. Surface methoxy species form the primary methanol decomposition products, which desorb partly by recombination as methanol at 200–300 K or decompose into CHx and possibly CO. The most reactive surfaces are mixed CuO–CuWO4 phase, with CuWO4 coverages 0.5–0.8 monolayer, thus pointing at the importance of oxide phase boundary sites. In a minority reaction channel, a small amount of formaldehyde is detected on the CuWO4 surface. The CuWO4 oxide phase becomes modified as a result of reduction and a morphology transition triggered by the methanol decomposition, but the pristine surface state can be recovered by a postoxidation treatment with oxygen.
中文翻译:

混合CuO–CuWO 4表面上的甲醇分解
通过在表面形成CuO(2×1)氧化物与吸附的(WO 3)3团簇,在Cu(110)表面制备了混合的CuO(2×1)–CuWO 4层。这些明确定义的CuO–CuWO 4对甲醇的吸附和分解表面之后是高分辨率X射线光电子能谱(XPS),高分辨率电子能量损失能谱(HREELS)和程序升温脱附(TPD)来评估分子表面种类及其浓度,而分子的状态通过扫描隧道显微镜(STM),低能电子衍射(LEED)和XPS对甲醇分解前后的表面氧化物相进行了表征。表面甲氧基物质形成主要的甲醇分解产物,部分分解成200-300 K的甲醇而解吸,或分解成CH x甚至可能分解成CO。反应性最大的表面是CuO–CuWO 4相和CuWO 4覆盖0.5-0.8单层,因此指出了氧化物相边界位点的重要性。在少数反应通道中,在CuWO 4表面检测到少量甲醛。由于还原和甲醇分解触发的形态转变,CuWO 4氧化物相被改性,但是可以通过用氧气进行后氧化处理来恢复原始的表面状态。
更新日期:2017-09-04
中文翻译:

混合CuO–CuWO 4表面上的甲醇分解
通过在表面形成CuO(2×1)氧化物与吸附的(WO 3)3团簇,在Cu(110)表面制备了混合的CuO(2×1)–CuWO 4层。这些明确定义的CuO–CuWO 4对甲醇的吸附和分解表面之后是高分辨率X射线光电子能谱(XPS),高分辨率电子能量损失能谱(HREELS)和程序升温脱附(TPD)来评估分子表面种类及其浓度,而分子的状态通过扫描隧道显微镜(STM),低能电子衍射(LEED)和XPS对甲醇分解前后的表面氧化物相进行了表征。表面甲氧基物质形成主要的甲醇分解产物,部分分解成200-300 K的甲醇而解吸,或分解成CH x甚至可能分解成CO。反应性最大的表面是CuO–CuWO 4相和CuWO 4覆盖0.5-0.8单层,因此指出了氧化物相边界位点的重要性。在少数反应通道中,在CuWO 4表面检测到少量甲醛。由于还原和甲醇分解触发的形态转变,CuWO 4氧化物相被改性,但是可以通过用氧气进行后氧化处理来恢复原始的表面状态。

































 京公网安备 11010802027423号
京公网安备 11010802027423号