当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Energetics of Baird aromaticity supported by inversion of photoexcited chiral [4n]annulene derivatives.
Nature Communications ( IF 14.7 ) Pub Date : 2017-08-24 , DOI: 10.1038/s41467-017-00382-1
Michihisa Ueda 1 , Kjell Jorner 2 , Young Mo Sung 3 , Tadashi Mori 4 , Qi Xiao 1 , Dongho Kim 3 , Henrik Ottosson 2 , Takuzo Aida 1, 5 , Yoshimitsu Itoh 1
Nature Communications ( IF 14.7 ) Pub Date : 2017-08-24 , DOI: 10.1038/s41467-017-00382-1
Michihisa Ueda 1 , Kjell Jorner 2 , Young Mo Sung 3 , Tadashi Mori 4 , Qi Xiao 1 , Dongho Kim 3 , Henrik Ottosson 2 , Takuzo Aida 1, 5 , Yoshimitsu Itoh 1
Affiliation
|
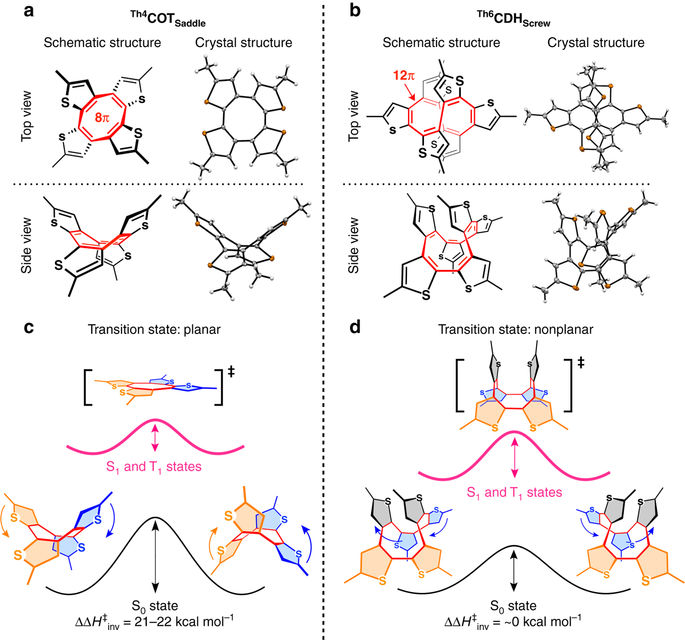
For the concept of aromaticity, energetic quantification is crucial. However, this has been elusive for excited-state (Baird) aromaticity. Here we report our serendipitous discovery of two nonplanar thiophene-fused chiral [4n]annulenes Th4 COT Saddle and Th6 CDH Screw , which by computational analysis turned out to be a pair of molecules suitable for energetic quantification of Baird aromaticity. Their enantiomers were separable chromatographically but racemized thermally, enabling investigation of the ring inversion kinetics. In contrast to Th6 CDH Screw , which inverts through a nonplanar transition state, the inversion of Th4 COT Saddle , progressing through a planar transition state, was remarkably accelerated upon photoexcitation. As predicted by Baird's theory, the planar conformation of Th4 COT Saddle is stabilized in the photoexcited state, thereby enabling lower activation enthalpy than that in the ground state. The lowering of the activation enthalpy, i.e., the energetic impact of excited-state aromaticity, was quantified experimentally to be as high as 21-22 kcal mol-1.Baird's rule applies to cyclic π-conjugated molecules in their excited state, yet a quantification of the involved energetics is elusive. Here, the authors show the ring inversion kinetics of two nonplanar and chiral [4n]annulenes to support Baird's rule from an energetic point of view.
中文翻译:

光激发手性[4n]轮烯衍生物的反转支持贝尔德芳香性的能量学。
对于芳香性的概念,能量量化至关重要。然而,这对于激发态(贝尔德)芳香性来说是难以捉摸的。在这里,我们报告了我们偶然发现的两种非平面噻吩稠合手性[4n]轮烯Th4 COT Saddle和Th6 CDH Screw ,通过计算分析证明它们是一对适合贝尔德芳香性能量定量的分子。它们的对映体可通过色谱分离,但可通过热外消旋化,从而能够研究环反转动力学。与通过非平面过渡态反转的Th6 CDH Screw相比,通过平面过渡态进行的Th4 COT Saddle的反转在光激发时显着加速。正如贝尔德理论所预测的, Th4 COT Saddle的平面构象在光激发态下是稳定的,从而能够实现比基态更低的活化焓。活化焓的降低,即激发态芳香性的能量影响,经实验量化高达 21-22 kcal mol -1 。贝尔德规则适用于处于激发态的环状 π 共轭分子,但所涉及的能量的量化是难以捉摸的。在这里,作者展示了两个非平面和手性[4n]轮烯的环反转动力学,从能量的角度支持贝尔德规则。
更新日期:2017-08-24
中文翻译:

光激发手性[4n]轮烯衍生物的反转支持贝尔德芳香性的能量学。
对于芳香性的概念,能量量化至关重要。然而,这对于激发态(贝尔德)芳香性来说是难以捉摸的。在这里,我们报告了我们偶然发现的两种非平面噻吩稠合手性[4n]轮烯Th4 COT Saddle和Th6 CDH Screw ,通过计算分析证明它们是一对适合贝尔德芳香性能量定量的分子。它们的对映体可通过色谱分离,但可通过热外消旋化,从而能够研究环反转动力学。与通过非平面过渡态反转的Th6 CDH Screw相比,通过平面过渡态进行的Th4 COT Saddle的反转在光激发时显着加速。正如贝尔德理论所预测的, Th4 COT Saddle的平面构象在光激发态下是稳定的,从而能够实现比基态更低的活化焓。活化焓的降低,即激发态芳香性的能量影响,经实验量化高达 21-22 kcal mol -1 。贝尔德规则适用于处于激发态的环状 π 共轭分子,但所涉及的能量的量化是难以捉摸的。在这里,作者展示了两个非平面和手性[4n]轮烯的环反转动力学,从能量的角度支持贝尔德规则。































 京公网安备 11010802027423号
京公网安备 11010802027423号