Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study.
The Lancet ( IF 98.4 ) Pub Date : 2017-07-19 , DOI: 10.1016/s1470-2045(17)30422-9 Michael J Overman 1 , Ray McDermott 2 , Joseph L Leach 3 , Sara Lonardi 4 , Heinz-Josef Lenz 5 , Michael A Morse 6 , Jayesh Desai 7 , Andrew Hill 8 , Michael Axelson 9 , Rebecca A Moss 9 , Monica V Goldberg 9 , Z Alexander Cao 9 , Jean-Marie Ledeine 10 , Gregory A Maglinte 9 , Scott Kopetz 1 , Thierry André 11
The Lancet ( IF 98.4 ) Pub Date : 2017-07-19 , DOI: 10.1016/s1470-2045(17)30422-9 Michael J Overman 1 , Ray McDermott 2 , Joseph L Leach 3 , Sara Lonardi 4 , Heinz-Josef Lenz 5 , Michael A Morse 6 , Jayesh Desai 7 , Andrew Hill 8 , Michael Axelson 9 , Rebecca A Moss 9 , Monica V Goldberg 9 , Z Alexander Cao 9 , Jean-Marie Ledeine 10 , Gregory A Maglinte 9 , Scott Kopetz 1 , Thierry André 11
Affiliation
|
BACKGROUND
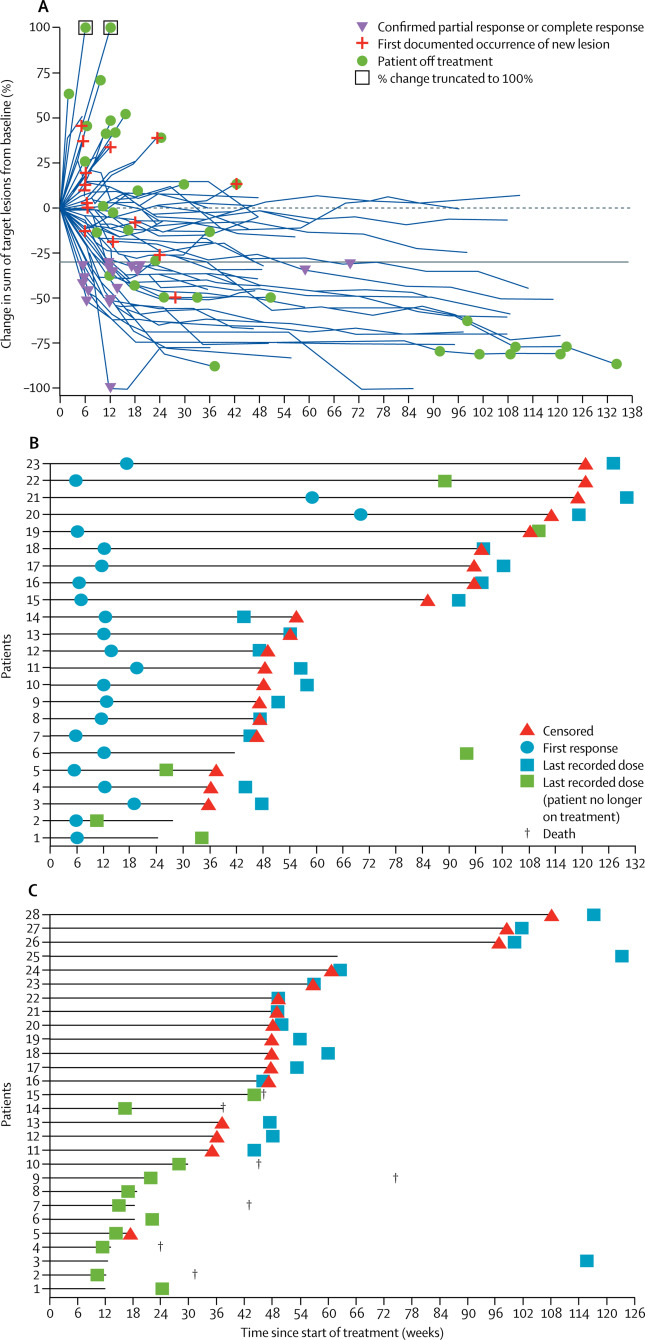
Metastatic DNA mismatch repair-deficient (dMMR)/microsatellite instability-high (MSI-H) colorectal cancer has a poor prognosis after treatment with conventional chemotherapy and exhibits high levels of tumour neoantigens, tumour-infiltrating lymphocytes, and checkpoint regulators. All of these features are associated with the response to PD-1 blockade in other tumour types. Therefore, we aimed to study nivolumab, a PD-1 immune checkpoint inhibitor, in patients with dMMR/MSI-H metastatic colorectal cancer.
METHODS
In this ongoing, multicentre, open-label, phase 2 trial, we enrolled adults (aged ≥18 years) with histologically confirmed recurrent or metastatic colorectal cancer locally assessed as dMMR/MSI-H from 31 sites (academic centres and hospitals) in eight countries (Australia, Belgium, Canada, France, Ireland, Italy, Spain, and the USA). Eligible patients had progressed on or after, or been intolerant of, at least one previous line of treatment, including a fluoropyrimidine and oxaliplatin or irinotecan. Patients were given 3 mg/kg nivolumab every 2 weeks until disease progression, death, unacceptable toxic effects, or withdrawal from study. The primary endpoint was investigator-assessed objective response as per Response Evaluation Criteria in Solid Tumors (version 1.1). All patients who received at least one dose of study drug were included in all analyses. This trial is registered with ClinicalTrials.gov, number NCT02060188.
FINDINGS
Of the 74 patients who were enrolled between March 12, 2014, and March 16, 2016, 40 (54%) had received three or more previous treatments. At a median follow-up of 12·0 months (IQR 8·6-18·0), 23 (31·1%, 95% CI 20·8-42·9) of 74 patients achieved an investigator-assessed objective response and 51 (69%, 57-79) patients had disease control for 12 weeks or longer. Median duration of response was not yet reached; all responders were alive, and eight had responses lasting 12 months or longer (Kaplan-Meier 12-month estimate 86%, 95% CI 62-95). The most common grade 3 or 4 drug-related adverse events were increased concentrations of lipase (six [8%]) and amylase (two [3%]). 23 (31%) patients died during the study; none of these deaths were deemed to be treatment related by the investigator.
INTERPRETATION
Nivolumab provided durable responses and disease control in pre-treated patients with dMMR/MSI-H metastatic colorectal cancer, and could be a new treatment option for these patients.
FUNDING
Bristol-Myers Squibb.
中文翻译:

Nivolumab 治疗转移性 DNA 错配修复缺陷或微卫星不稳定性高结直肠癌患者 (CheckMate 142):一项开放标签、多中心、2 期研究。
背景转移性DNA错配修复缺陷(dMMR)/微卫星不稳定性高(MSI-H)结直肠癌在常规化疗后预后不良,并表现出高水平的肿瘤新抗原、肿瘤浸润淋巴细胞和检查点调节因子。所有这些特征都与其他肿瘤类型对 PD-1 阻断的反应相关。因此,我们的目的是在 dMMR/MSI-H 转移性结直肠癌患者中研究纳武单抗(一种 PD-1 免疫检查点抑制剂)。方法 在这项正在进行的、多中心、开放标签、2 期试验中,我们从 31 个地点(学术中心和医院)招募了经组织学证实的复发性或转移性结直肠癌并局部评估为 dMMR/MSI-H 的成年人(年龄≥18 岁)。八个国家(澳大利亚、比利时、加拿大、法国、爱尔兰、意大利、西班牙和美国)。符合条件的患者在至少一种先前的治疗(包括氟嘧啶和奥沙利铂或伊立替康)期间或之后出现进展,或不耐受。患者每两周接受 3 mg/kg 纳武单抗治疗,直至疾病进展、死亡、出现不可接受的毒性反应或退出研究。主要终点是研究者根据实体瘤疗效评估标准(1.1 版)评估的客观疗效。所有接受至少一剂研究药物的患者均纳入所有分析中。该试验已在 ClinicalTrials.gov 注册,编号为 NCT02060188。结果 在 2014 年 3 月 12 日至 2016 年 3 月 16 日期间入组的 74 名患者中,40 名 (54%) 先前接受过三种或以上治疗。中位随访时间为 12·0 个月 (IQR 8·6-18·0),74 名患者中有 23 名 (31·1%,95% CI 20·8-42·9) 达到了研究者评估的客观缓解51 名(69%,57-79)名患者的疾病控制时间达到或超过 12 周。尚未达到中位缓解持续时间;所有响应者均存活,其中 8 名响应持续 12 个月或更长时间(Kaplan-Meier 12 个月估计 86%,95% CI 62-95)。最常见的 3 级或 4 级药物相关不良事件是脂肪酶(6 例 [8%])和淀粉酶(2 例 [3%])浓度升高。23 名 (31%) 患者在研究期间死亡;调查人员认为这些死亡均与治疗无关。解释 纳武单抗为经过治疗的 dMMR/MSI-H 转移性结直肠癌患者提供了持久的缓解和疾病控制,并且可能成为这些患者的新治疗选择。资助百时美施贵宝。
更新日期:2017-08-10
中文翻译:

Nivolumab 治疗转移性 DNA 错配修复缺陷或微卫星不稳定性高结直肠癌患者 (CheckMate 142):一项开放标签、多中心、2 期研究。
背景转移性DNA错配修复缺陷(dMMR)/微卫星不稳定性高(MSI-H)结直肠癌在常规化疗后预后不良,并表现出高水平的肿瘤新抗原、肿瘤浸润淋巴细胞和检查点调节因子。所有这些特征都与其他肿瘤类型对 PD-1 阻断的反应相关。因此,我们的目的是在 dMMR/MSI-H 转移性结直肠癌患者中研究纳武单抗(一种 PD-1 免疫检查点抑制剂)。方法 在这项正在进行的、多中心、开放标签、2 期试验中,我们从 31 个地点(学术中心和医院)招募了经组织学证实的复发性或转移性结直肠癌并局部评估为 dMMR/MSI-H 的成年人(年龄≥18 岁)。八个国家(澳大利亚、比利时、加拿大、法国、爱尔兰、意大利、西班牙和美国)。符合条件的患者在至少一种先前的治疗(包括氟嘧啶和奥沙利铂或伊立替康)期间或之后出现进展,或不耐受。患者每两周接受 3 mg/kg 纳武单抗治疗,直至疾病进展、死亡、出现不可接受的毒性反应或退出研究。主要终点是研究者根据实体瘤疗效评估标准(1.1 版)评估的客观疗效。所有接受至少一剂研究药物的患者均纳入所有分析中。该试验已在 ClinicalTrials.gov 注册,编号为 NCT02060188。结果 在 2014 年 3 月 12 日至 2016 年 3 月 16 日期间入组的 74 名患者中,40 名 (54%) 先前接受过三种或以上治疗。中位随访时间为 12·0 个月 (IQR 8·6-18·0),74 名患者中有 23 名 (31·1%,95% CI 20·8-42·9) 达到了研究者评估的客观缓解51 名(69%,57-79)名患者的疾病控制时间达到或超过 12 周。尚未达到中位缓解持续时间;所有响应者均存活,其中 8 名响应持续 12 个月或更长时间(Kaplan-Meier 12 个月估计 86%,95% CI 62-95)。最常见的 3 级或 4 级药物相关不良事件是脂肪酶(6 例 [8%])和淀粉酶(2 例 [3%])浓度升高。23 名 (31%) 患者在研究期间死亡;调查人员认为这些死亡均与治疗无关。解释 纳武单抗为经过治疗的 dMMR/MSI-H 转移性结直肠癌患者提供了持久的缓解和疾病控制,并且可能成为这些患者的新治疗选择。资助百时美施贵宝。

































 京公网安备 11010802027423号
京公网安备 11010802027423号