当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric Nitrone Synthesis via Ligand-Enabled Copper-Catalyzed Cope-Type Hydroamination of Cyclopropene with Oxime
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-08-17 , DOI: 10.1021/jacs.7b06523 Zhanyu Li 1 , Jinbo Zhao 1 , Baozhen Sun 1 , Tingting Zhou 1 , Mingzhu Liu 1 , Shuang Liu 1 , Mengru Zhang 1 , Qian Zhang 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-08-17 , DOI: 10.1021/jacs.7b06523 Zhanyu Li 1 , Jinbo Zhao 1 , Baozhen Sun 1 , Tingting Zhou 1 , Mingzhu Liu 1 , Shuang Liu 1 , Mengru Zhang 1 , Qian Zhang 1
Affiliation
|
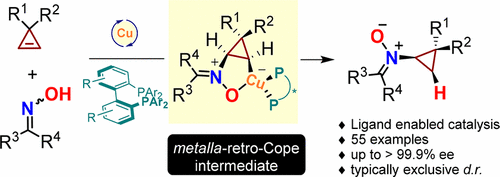
We report realization of the first enantioselective Cope-type hydroamination of oximes for asymmetric nitrone synthesis. The ligand promoted asymmetric cyclopropene "hydronitronylation" process employs a Cu-based catalytic system and readily available starting materials, operates under mild conditions and displays broad scope and exceptionally high enantio- and diastereocontrol. Preliminary mechanistic studies corroborate a CuI-catalytic profile featuring an olefin metalla-retro-Cope aminocupration process as the key C-N bond forming event. This conceptually novel reactivity enables the first example of highly enantioselective catalytic nitrone formation process and will likely spur further developments that may significantly expedite chiral nitrone synthesis.
中文翻译:

通过配体启用的铜催化环丙烯与肟的 Cope 型加氢胺化反应合成不对称硝酮
我们报告了用于不对称硝酮合成的第一个对映选择性 Cope 型肟加氢胺化的实现。配体促进的不对称环丙烯“加氢硝基化”过程采用基于铜的催化系统和容易获得的起始材料,在温和的条件下运行,显示出广泛的范围和极高的对映和非对映控制。初步机理研究证实了 CuI 催化特性,其特征是烯烃金属-逆-Cope 氨基铜化过程是关键的 CN 键形成事件。这种概念上新颖的反应性使高度对映选择性催化硝酮形成过程的第一个例子成为可能,并且可能会刺激进一步的发展,从而显着加快手性硝酮的合成。
更新日期:2017-08-17
中文翻译:

通过配体启用的铜催化环丙烯与肟的 Cope 型加氢胺化反应合成不对称硝酮
我们报告了用于不对称硝酮合成的第一个对映选择性 Cope 型肟加氢胺化的实现。配体促进的不对称环丙烯“加氢硝基化”过程采用基于铜的催化系统和容易获得的起始材料,在温和的条件下运行,显示出广泛的范围和极高的对映和非对映控制。初步机理研究证实了 CuI 催化特性,其特征是烯烃金属-逆-Cope 氨基铜化过程是关键的 CN 键形成事件。这种概念上新颖的反应性使高度对映选择性催化硝酮形成过程的第一个例子成为可能,并且可能会刺激进一步的发展,从而显着加快手性硝酮的合成。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号