当前位置:
X-MOL 学术
›
J. Membr. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Current-voltage characteristic of anion-exchange membrane in monosodium phosphate solution. Modelling and experiment
Journal of Membrane Science ( IF 8.4 ) Pub Date : 2017-11-01 , DOI: 10.1016/j.memsci.2017.08.002 E.D. Belashova , N.D. Pismenskaya , V.V. Nikonenko , P. Sistat , G. Pourcelly
Journal of Membrane Science ( IF 8.4 ) Pub Date : 2017-11-01 , DOI: 10.1016/j.memsci.2017.08.002 E.D. Belashova , N.D. Pismenskaya , V.V. Nikonenko , P. Sistat , G. Pourcelly
|
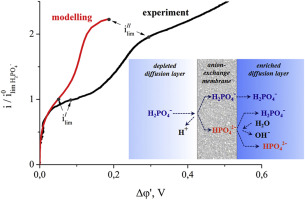
Abstract A current-voltage characteristic of a Neosepta AMX membrane in a monosodium phosphate solution was investigated experimentally and theoretically. It is shown that the experimental curve has two sections of the overlimiting "plateau" and, accordingly, two limiting current densities. This feature is not observed in the presence of electrolytes, such as sodium chloride. For a better understanding of the phenomenon, a one-dimensional three-layer steady-state transfer model taking into account the possibility of ampholyte species transformation depending on the local pH is developed. Comparison of the calculations with the experimental data and the interpretation of the current-voltage characteristic are made. The first limiting current is due to the vanishing of single-charged phosphate anions in the solution at the membrane surface. The following growth of current density is caused by the protons generated during the transformation of the single-charged phosphate anions ( H 2 P O 4 − ) into the double-charged ones ( H P O 4 2 − ) when entering the membrane. This transformation is due to the increase in pH of the AMX internal solution with increasing current density. The second limiting current is observed when the membrane is almost completely converted into the form of double-charged phosphate anions. In this state the proton flux generated in the H 2 P O 4 − – H P O 4 2 − transformation at the depleted solution/membrane interface approaches its maximum.
中文翻译:

磷酸一钠溶液中阴离子交换膜的电流-电压特性。建模与实验
摘要 通过实验和理论研究了Neosepta AMX 膜在磷酸一钠溶液中的电流-电压特性。结果表明,实验曲线有两段超限“平台”,因此有两个极限电流密度。在存在电解质(例如氯化钠)时未观察到此特征。为了更好地理解这一现象,开发了一个一维三层稳态转移模型,考虑到两性电解质物种转化的可能性取决于局部 pH 值。计算与实验数据的比较和电流-电压特性的解释。第一个极限电流是由于膜表面溶液中单电荷磷酸根阴离子的消失。电流密度的以下增长是由进入膜时单电荷磷酸根阴离子 (H 2 PO 4 - ) 转化为双电荷阴离子 (HPO 4 2 - ) 期间产生的质子引起的。这种转变是由于 AMX 内部溶液的 pH 值随着电流密度的增加而增加。当膜几乎完全转化为双电荷磷酸根阴离子时,观察到第二个极限电流。在这种状态下,在耗尽溶液/膜界面处的 H 2 PO 4 - – HPO 4 2 - 转化中产生的质子通量接近其最大值。
更新日期:2017-11-01
中文翻译:

磷酸一钠溶液中阴离子交换膜的电流-电压特性。建模与实验
摘要 通过实验和理论研究了Neosepta AMX 膜在磷酸一钠溶液中的电流-电压特性。结果表明,实验曲线有两段超限“平台”,因此有两个极限电流密度。在存在电解质(例如氯化钠)时未观察到此特征。为了更好地理解这一现象,开发了一个一维三层稳态转移模型,考虑到两性电解质物种转化的可能性取决于局部 pH 值。计算与实验数据的比较和电流-电压特性的解释。第一个极限电流是由于膜表面溶液中单电荷磷酸根阴离子的消失。电流密度的以下增长是由进入膜时单电荷磷酸根阴离子 (H 2 PO 4 - ) 转化为双电荷阴离子 (HPO 4 2 - ) 期间产生的质子引起的。这种转变是由于 AMX 内部溶液的 pH 值随着电流密度的增加而增加。当膜几乎完全转化为双电荷磷酸根阴离子时,观察到第二个极限电流。在这种状态下,在耗尽溶液/膜界面处的 H 2 PO 4 - – HPO 4 2 - 转化中产生的质子通量接近其最大值。













































 京公网安备 11010802027423号
京公网安备 11010802027423号