当前位置:
X-MOL 学术
›
J. Mater. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Persistent luminescent nanoparticles as energy mediators for enhanced photodynamic therapy with fractionated irradiation
Journal of Materials Chemistry B ( IF 6.1 ) Pub Date : 2017-06-13 00:00:00 , DOI: 10.1039/c7tb00950j
Jing Wang 1, 2, 3, 4, 5 , Yujie Li 6, 7, 8, 9, 10 , Rihua Mao 4, 5, 11, 12, 13 , Yong Wang 4, 5, 11, 12, 13 , Xiuping Yan 6, 7, 8, 9, 10 , Jun Liu 1, 2, 3, 4, 5
Journal of Materials Chemistry B ( IF 6.1 ) Pub Date : 2017-06-13 00:00:00 , DOI: 10.1039/c7tb00950j
Jing Wang 1, 2, 3, 4, 5 , Yujie Li 6, 7, 8, 9, 10 , Rihua Mao 4, 5, 11, 12, 13 , Yong Wang 4, 5, 11, 12, 13 , Xiuping Yan 6, 7, 8, 9, 10 , Jun Liu 1, 2, 3, 4, 5
Affiliation
|
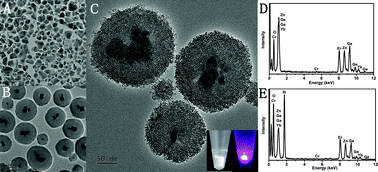
The excitation wavelengths of most porphyrin-based photosensitizers are in the ultraviolet (UV) spectrum. Prolonged irradiation of living cells and tissues with UV light during the clinical application of photodynamic therapy (PDT) may cause DNA damage and cell death. Here, we report a novel persistent-luminescent nanoparticle (PLNP)-based PDT approach that uses the afterglow property of PLNPs to greatly reduce the dose of UV light while maintaining the desired cancer suppression effect. Multifunctional PLNPs coated with mesoporous silica layers and subsequently conjugated to a photosensitizer were evaluated. These nanoconjugates showed high colloidal stability and biocompatibility. Furthermore, they generated a moderate amount of 1O2 through efficient energy transfer from the nanoparticle to the photosensitizer, which can efficiently damage cancer cells. In addition to their UV-excited luminescence, PLNPs also exhibited a long-lasting luminescence afterglow. Thus, PLNPs can serve as a persistent light source for PDT activation after excitation by an external light source is stopped. When fractionated light was used for excitation instead of continuous light at equivalent irradiation doses, confocal microscopy revealed that the photosensitizer-conjugated PLNPs showed a significantly enhanced cancer cell killing ability. Moreover, quantitative flow cytometry showed that fractionated light irradiation (60 s/100 s on/off cycle) produced up to ten times more cancer cell apoptosis/necrosis than the same dose of continuous light irradiation did. These results indicate that photosensitizer-conjugated PLNPs combined with fractionated irradiation show good potential for low-dose UV-mediated PDT activation.
中文翻译:

持久发光纳米粒子作为能量介质,用于分次辐射增强光动力治疗
大多数基于卟啉的光敏剂的激发波长在紫外线(UV)光谱中。在光动力疗法(PDT)的临床应用过程中,用紫外线长时间照射活细胞和组织可能会导致DNA损伤和细胞死亡。在这里,我们报告了一种新颖的基于持久发光纳米粒子(PLNP)的PDT方法,该方法利用PLNP的余辉特性来大大降低紫外线的剂量,同时保持所需的癌症抑制效果。评估了涂覆有介孔二氧化硅层并随后与光敏剂偶联的多功能PLNP。这些纳米共轭物显示出高的胶体稳定性和生物相容性。此外,它们产生了适量的1 O 2通过从纳米颗粒到光敏剂的有效能量转移,可以有效地破坏癌细胞。除了其紫外线激发的发光外,PLNP还显示出持久的发光余辉。因此,在停止外部光源的激励之后,PLNP可以用作PDT激活的持久光源。当使用分光代替等效光照射下的连续光激发时,共聚焦显微镜显示光敏剂偶联的PLNPs显着增强了癌细胞的杀伤能力。此外,定量流式细胞仪显示分光照射(60 s / 100 s开/关周期)产生的癌细胞凋亡/坏死率是相同剂量连续光照射的十倍之多。
更新日期:2017-07-30
中文翻译:

持久发光纳米粒子作为能量介质,用于分次辐射增强光动力治疗
大多数基于卟啉的光敏剂的激发波长在紫外线(UV)光谱中。在光动力疗法(PDT)的临床应用过程中,用紫外线长时间照射活细胞和组织可能会导致DNA损伤和细胞死亡。在这里,我们报告了一种新颖的基于持久发光纳米粒子(PLNP)的PDT方法,该方法利用PLNP的余辉特性来大大降低紫外线的剂量,同时保持所需的癌症抑制效果。评估了涂覆有介孔二氧化硅层并随后与光敏剂偶联的多功能PLNP。这些纳米共轭物显示出高的胶体稳定性和生物相容性。此外,它们产生了适量的1 O 2通过从纳米颗粒到光敏剂的有效能量转移,可以有效地破坏癌细胞。除了其紫外线激发的发光外,PLNP还显示出持久的发光余辉。因此,在停止外部光源的激励之后,PLNP可以用作PDT激活的持久光源。当使用分光代替等效光照射下的连续光激发时,共聚焦显微镜显示光敏剂偶联的PLNPs显着增强了癌细胞的杀伤能力。此外,定量流式细胞仪显示分光照射(60 s / 100 s开/关周期)产生的癌细胞凋亡/坏死率是相同剂量连续光照射的十倍之多。

































 京公网安备 11010802027423号
京公网安备 11010802027423号