当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrochemical reactions in fluoride-ion batteries: mechanistic insights from pair distribution function analysis
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2017-07-05 00:00:00 , DOI: 10.1039/c7ta04005a Antonin Grenier 1, 2, 3, 4, 5 , Ana-Gabriela Porras-Gutierrez 1, 2, 3, 4, 5 , Henri Groult 1, 2, 3, 4, 5 , Kevin A. Beyer 6, 7, 8, 9, 10 , Olaf J. Borkiewicz 6, 7, 8, 9, 10 , Karena W. Chapman 6, 7, 8, 9, 10 , Damien Dambournet 1, 2, 3, 4, 5
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2017-07-05 00:00:00 , DOI: 10.1039/c7ta04005a Antonin Grenier 1, 2, 3, 4, 5 , Ana-Gabriela Porras-Gutierrez 1, 2, 3, 4, 5 , Henri Groult 1, 2, 3, 4, 5 , Kevin A. Beyer 6, 7, 8, 9, 10 , Olaf J. Borkiewicz 6, 7, 8, 9, 10 , Karena W. Chapman 6, 7, 8, 9, 10 , Damien Dambournet 1, 2, 3, 4, 5
Affiliation
|
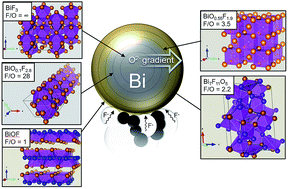
Detailed analysis of electrochemical reactions occurring in rechargeable Fluoride-Ion Batteries (FIBs) is provided by means of synchrotron X-ray diffraction (XRD) and Pair Distribution Function (PDF) analysis. In this study, a symmetrical cell containing Bi–BiF3 composite electrodes was characterized after three cycles. As for Li conversion-based electrodes, our study confirms that a multi-electron redox process occurs between Bi metal and BiF3. XRD and PDF analyses show that multiple Bi oxyfluoride phases are formed due to the presence of Bi oxide in the initial Bi electrode and/or Bi oxyfluoride in the initial BiF3 electrode. Quantitative PDF analysis suggests that oxygen does not migrate through the electrolyte and that the fluoride ion is the sole charge transfer anion. Hence, we showed that oxide species do not prevent the electrochemical reactions, opening the path for the study of metal oxyfluorides as active materials for FIBs.
中文翻译:

氟离子电池中的电化学反应:对对分布函数分析的机理见解
借助于同步加速器X射线衍射(XRD)和成对分布函数(PDF)分析,详细分析了可充电氟离子电池(FIB)中发生的电化学反应。在这项研究中,经过三个循环后,对包含Bi–BiF 3复合电极的对称电池进行了表征。对于基于Li转换的电极,我们的研究证实了Bi金属和BiF 3之间发生了多电子氧化还原过程。XRD和PDF分析表明,由于初始Bi电极中存在Bi氧化物,形成了多个Bi氟氧化物相。和/或初始BiF 3中的氟化氧双电极。PDF的定量分析表明,氧不会通过电解质迁移,而氟离子是唯一的电荷转移阴离子。因此,我们证明了氧化物不会阻止电化学反应,为研究金属氧氟化物作为FIB的活性材料开辟了道路。
更新日期:2017-07-21
中文翻译:

氟离子电池中的电化学反应:对对分布函数分析的机理见解
借助于同步加速器X射线衍射(XRD)和成对分布函数(PDF)分析,详细分析了可充电氟离子电池(FIB)中发生的电化学反应。在这项研究中,经过三个循环后,对包含Bi–BiF 3复合电极的对称电池进行了表征。对于基于Li转换的电极,我们的研究证实了Bi金属和BiF 3之间发生了多电子氧化还原过程。XRD和PDF分析表明,由于初始Bi电极中存在Bi氧化物,形成了多个Bi氟氧化物相。和/或初始BiF 3中的氟化氧双电极。PDF的定量分析表明,氧不会通过电解质迁移,而氟离子是唯一的电荷转移阴离子。因此,我们证明了氧化物不会阻止电化学反应,为研究金属氧氟化物作为FIB的活性材料开辟了道路。











































 京公网安备 11010802027423号
京公网安备 11010802027423号