当前位置:
X-MOL 学术
›
Adv. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Disordered Conformation with Low Pii Helix in Phosphoproteins Orchestrates Biomimetic Apatite Formation
Advanced Materials ( IF 27.4 ) Pub Date : 2017-07-17 , DOI: 10.1002/adma.201701629 Melika Sarem 1, 2 , Steffen Lüdeke 3 , Ralf Thomann 4 , Pavel Salavei 5 , Zhaoyong Zou 6 , Wouter Habraken 6 , Admir Masic 7 , V. Prasad Shastri 1, 2
Advanced Materials ( IF 27.4 ) Pub Date : 2017-07-17 , DOI: 10.1002/adma.201701629 Melika Sarem 1, 2 , Steffen Lüdeke 3 , Ralf Thomann 4 , Pavel Salavei 5 , Zhaoyong Zou 6 , Wouter Habraken 6 , Admir Masic 7 , V. Prasad Shastri 1, 2
Affiliation
|
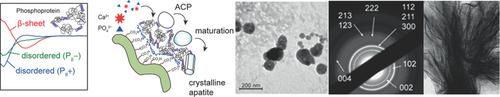
The interplay between noncollagenous proteins and biomineralization is widely accepted, yet the contribution of their secondary structure in mineral formation remains to be clarified. This study demonstrates a role for phosvitin, an intrinsically disordered phosphoprotein, in chick embryo skeletal development, and using circular dichroism and matrix least‐squares Henderson–Hasselbalch global fitting, unravels three distinct pH‐dependent secondary structures in phosvitin. By sequestering phosvitin on a biomimetic 3D insoluble cationic framework at defined pHs, access is gained to phosvitin in various conformational states. Induction of biomimetic mineralization at near physiological conditions reveals that a disordered secondary structure with a low content of PII helix is remarkably efficient at promoting calcium adsorption, and results in the formation of biomimetic hydroxyapatite through an amorphous calcium phosphate precursor. By extending this finding to phosphorylated full‐length human recombinant dentin matrix protein‐1 (17‐513 AA), this bioinspired approach provides compelling evidence for the role of a disordered secondary structure in phosphoproteins in bone‐like apatite formation.
中文翻译:

磷蛋白中低Pii螺旋的紊乱构象策划了仿生磷灰石的形成
非胶原蛋白与生物矿化之间的相互作用已被广泛接受,但其二级结构在矿物形成中的作用仍有待阐明。这项研究证明了磷光蛋白(一种内在失调的磷蛋白)在雏鸡骨骼发育中的作用,并使用圆二色性和矩阵最小二乘法Henderson-Hasselbalch全局拟合,揭示了磷光蛋白中三个不同的pH依赖性二级结构。通过在限定的pH值下在仿生的3D不溶性阳离子骨架上隔离磷光蛋白,可以获得各种构象状态的磷光蛋白。在接近生理条件的条件下诱导仿生矿化作用表明,二级结构紊乱,P II含量低螺旋在促进钙吸附方面非常有效,并导致通过无定形磷酸钙前体形成仿生羟基磷灰石。通过将这一发现扩展到磷酸化的全长人类重组牙本质基质蛋白-1(17-513 AA),这种受生物启发的方法提供了令人信服的证据,证明无序二级结构在磷蛋白中的作用类似于骨磷灰石的形成。
更新日期:2017-07-17
中文翻译:

磷蛋白中低Pii螺旋的紊乱构象策划了仿生磷灰石的形成
非胶原蛋白与生物矿化之间的相互作用已被广泛接受,但其二级结构在矿物形成中的作用仍有待阐明。这项研究证明了磷光蛋白(一种内在失调的磷蛋白)在雏鸡骨骼发育中的作用,并使用圆二色性和矩阵最小二乘法Henderson-Hasselbalch全局拟合,揭示了磷光蛋白中三个不同的pH依赖性二级结构。通过在限定的pH值下在仿生的3D不溶性阳离子骨架上隔离磷光蛋白,可以获得各种构象状态的磷光蛋白。在接近生理条件的条件下诱导仿生矿化作用表明,二级结构紊乱,P II含量低螺旋在促进钙吸附方面非常有效,并导致通过无定形磷酸钙前体形成仿生羟基磷灰石。通过将这一发现扩展到磷酸化的全长人类重组牙本质基质蛋白-1(17-513 AA),这种受生物启发的方法提供了令人信服的证据,证明无序二级结构在磷蛋白中的作用类似于骨磷灰石的形成。






























 京公网安备 11010802027423号
京公网安备 11010802027423号