当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural Basis for Aza-Glycine Stabilization of Collagen
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-07-06 00:00:00 , DOI: 10.1021/jacs.7b03398 Alexander J. Kasznel 1 , Yitao Zhang 1 , Yang Hai 1 , David M. Chenoweth 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-07-06 00:00:00 , DOI: 10.1021/jacs.7b03398 Alexander J. Kasznel 1 , Yitao Zhang 1 , Yang Hai 1 , David M. Chenoweth 1
Affiliation
|
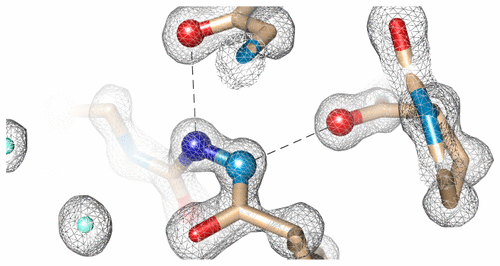
Previously, we have demonstrated that replacement of the strictly conserved glycine in collagen with aza-glycine provides a general solution for stabilizing triple helical collagen peptides.1,2 The additional hydrogen bond and conformational constraints provided by aza-glycine increases the thermal stability and rate of folding in collagen peptides composed of Pro-Hyp-Gly triplet repeats, allowing for truncation to the smallest self-assembling peptide systems observed to date. Here we show that aza-glycine substitution enhances the stability of an arginine-containing collagen peptide and provide a structural basis for this stabilization with an atomic resolution crystal structure. These results demonstrate that a single nitrogen atom substitution for a glycine alpha-carbon increases the peptide’s triple helix melting temperature by 8.6 °C. Furthermore, we provide the first structural basis for stabilization of triple helical collagen peptides containing aza-glycine and we demonstrate that minimal alteration to the peptide backbone conformation occurs with aza-glycine incorporation.
中文翻译:

氮杂-甘氨酸稳定胶原蛋白的结构基础
以前,我们已经证明了用aza-甘氨酸代替胶原蛋白中严格保守的甘氨酸为稳定三螺旋胶原蛋白肽提供了一个通用的解决方案。1,2aza-甘氨酸提供的额外氢键和构象约束条件提高了热稳定性和速率可折叠由Pro-Hyp-Gly三联体重复组成的胶原蛋白肽,从而可截短至迄今为止观察到的最小的自组装肽系统。在这里,我们显示了氮杂甘氨酸取代增强了含精氨酸胶原蛋白肽的稳定性,并为这种稳定提供了具有原子分辨率晶体结构的结构基础。这些结果表明,单个氮原子取代甘氨酸α-碳会使肽的三重螺旋熔化温度增加8.6°C。
更新日期:2017-07-08
中文翻译:

氮杂-甘氨酸稳定胶原蛋白的结构基础
以前,我们已经证明了用aza-甘氨酸代替胶原蛋白中严格保守的甘氨酸为稳定三螺旋胶原蛋白肽提供了一个通用的解决方案。1,2aza-甘氨酸提供的额外氢键和构象约束条件提高了热稳定性和速率可折叠由Pro-Hyp-Gly三联体重复组成的胶原蛋白肽,从而可截短至迄今为止观察到的最小的自组装肽系统。在这里,我们显示了氮杂甘氨酸取代增强了含精氨酸胶原蛋白肽的稳定性,并为这种稳定提供了具有原子分辨率晶体结构的结构基础。这些结果表明,单个氮原子取代甘氨酸α-碳会使肽的三重螺旋熔化温度增加8.6°C。











































 京公网安备 11010802027423号
京公网安备 11010802027423号