Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Post-Translational Sortase-Mediated Attachment of High-Strength Force Spectroscopy Handles
ACS Omega ( IF 3.7 ) Pub Date : 2017-06-30 00:00:00 , DOI: 10.1021/acsomega.7b00478 Ellis Durner 1 , Wolfgang Ott 1 , Michael A Nash 2, 3 , Hermann E Gaub 1
ACS Omega ( IF 3.7 ) Pub Date : 2017-06-30 00:00:00 , DOI: 10.1021/acsomega.7b00478 Ellis Durner 1 , Wolfgang Ott 1 , Michael A Nash 2, 3 , Hermann E Gaub 1
Affiliation
|
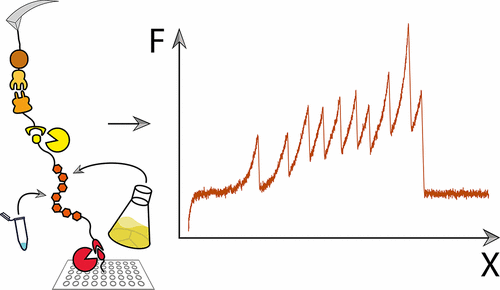
Single-molecule force spectroscopy greatly benefits from site-specific surface immobilization and specific probing with a functionalized cantilever. Here, we describe a streamlined approach to such experiments by covalently attaching mechanically stable receptors onto proteins of interest (POI) to improve pickup efficiency and specificity. This platform provides improved throughput, allows precise control over the pulling geometry, and allows for multiple constructs to be probed with the same ligand-modified cantilever. We employ two orthogonal enzymatic ligation reactions [sortase and phosphopantetheinyl transferase (Sfp)] to covalently immobilize POI to a pegylated surface and to subsequently ligate the POI to a mechanically stable dockerin domain at the protein’s C-terminus for use as a high-strength pulling handle. Our configuration permits expression and folding of the POI to proceed independently from the mechanically stable receptor used for specific probing and requires only two short terminal peptide sequences (i.e., ybbR-tag and sortase C-tag). We applied this system successfully to proteins expressed using in vitro transcription and translation reactions without a protein purification step and to purified proteins expressed in Escherichia coli.
中文翻译:

翻译后分选酶介导的高强度力谱手柄的附着
单分子力谱极大地受益于位点特异性表面固定和功能化悬臂的特异性探测。在这里,我们描述了此类实验的简化方法,通过将机械稳定的受体共价连接到感兴趣的蛋白质(POI)上以提高拾取效率和特异性。该平台提供了改进的吞吐量,允许精确控制牵引几何形状,并允许使用相同的配体修饰悬臂探测多个结构。我们采用两个正交酶连接反应 [分选酶和磷酸泛酰基转移酶 (Sfp)] 将 POI 共价固定到聚乙二醇化表面,然后将 POI 连接到蛋白质 C 末端的机械稳定的 dockerin 结构域,用作高强度牵引处理。我们的配置允许 POI 的表达和折叠独立于用于特定探测的机械稳定受体进行,并且仅需要两个短末端肽序列(即 ybbR 标签和分选酶 C 标签)。我们成功地将这个系统应用于使用体外转录和翻译反应表达的蛋白质(无需蛋白质纯化步骤)以及在大肠杆菌中表达的纯化蛋白质。
更新日期:2017-06-30
中文翻译:

翻译后分选酶介导的高强度力谱手柄的附着
单分子力谱极大地受益于位点特异性表面固定和功能化悬臂的特异性探测。在这里,我们描述了此类实验的简化方法,通过将机械稳定的受体共价连接到感兴趣的蛋白质(POI)上以提高拾取效率和特异性。该平台提供了改进的吞吐量,允许精确控制牵引几何形状,并允许使用相同的配体修饰悬臂探测多个结构。我们采用两个正交酶连接反应 [分选酶和磷酸泛酰基转移酶 (Sfp)] 将 POI 共价固定到聚乙二醇化表面,然后将 POI 连接到蛋白质 C 末端的机械稳定的 dockerin 结构域,用作高强度牵引处理。我们的配置允许 POI 的表达和折叠独立于用于特定探测的机械稳定受体进行,并且仅需要两个短末端肽序列(即 ybbR 标签和分选酶 C 标签)。我们成功地将这个系统应用于使用体外转录和翻译反应表达的蛋白质(无需蛋白质纯化步骤)以及在大肠杆菌中表达的纯化蛋白质。

































 京公网安备 11010802027423号
京公网安备 11010802027423号