当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Intestinal microbial dysbiosis aggravates the progression of Alzheimer's disease in Drosophila.
Nature Communications ( IF 14.7 ) Pub Date : 2017-06-20 , DOI: 10.1038/s41467-017-00040-6 Shih-Cheng Wu , Zih-Syuan Cao , Kuo-Ming Chang , Jyh-Lyh Juang
Nature Communications ( IF 14.7 ) Pub Date : 2017-06-20 , DOI: 10.1038/s41467-017-00040-6 Shih-Cheng Wu , Zih-Syuan Cao , Kuo-Ming Chang , Jyh-Lyh Juang
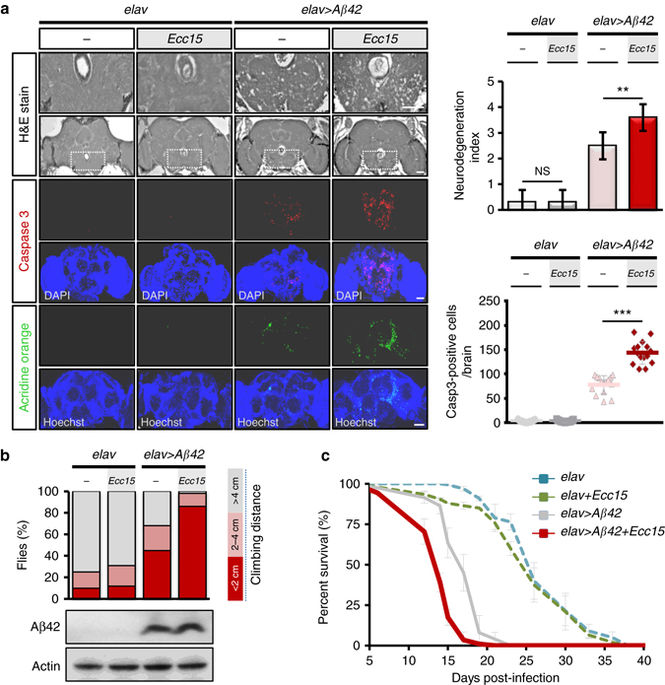
|
Neuroinflammation caused by local deposits of Aβ42 in the brain is key for the pathogenesis and progression of Alzheimer's disease. However, inflammation in the brain is not always a response to local primary insults. Gut microbiota dysbiosis, which is recently emerging as a risk factor for psychiatric disorders, can also initiate a brain inflammatory response. It still remains unclear however, whether enteric dysbiosis also contributes to Alzheimer's disease. Here we show that in a Drosophila Alzheimer's disease model, enterobacteria infection exacerbated progression of Alzheimer's disease by promoting immune hemocyte recruitment to the brain, thereby provoking TNF-JNK mediated neurodegeneration. Genetic depletion of hemocytes attenuates neuroinflammation and alleviated neurodegeneration. We further found that enteric infection increases the motility of the hemocytes, making them more readily attracted to the brain with an elevated oxidative stress status. This work highlights the importance of gut-brain crosstalk as a fundamental regulatory system in modulating Alzheimer's disease neurodegeneration.Emerging evidence suggests that gut microbiota influences immune function in the brain and may play a role in neurological diseases. Here, the authors offer in vivo evidence from a Drosophila model that supports a role for gut microbiota in modulating the progression of Alzheimer's disease.
中文翻译:

肠道微生物营养不良会加剧果蝇中阿尔茨海默氏病的进展。
Aβ42局部沉积引起的神经炎症在大脑中,阿尔茨海默氏病的发病机制和发展至关重要。然而,脑部炎症并不总是对局部原发性损伤的反应。肠道菌群失调最近已成为精神疾病的危险因素,它也可以引发脑部炎症反应。然而,肠营养不良是否也导致阿尔茨海默氏病仍不清楚。在这里我们表明,在果蝇阿尔茨海默氏病模型中,肠杆菌感染通过促进免疫血细胞募集到大脑而加剧了TNF-JNK介导的神经变性,从而加剧了阿尔茨海默氏病的进展。血细胞的遗传耗竭减弱了神经炎症并减轻了神经变性。我们进一步发现,肠感染会增加血细胞的运动能力,使它们更容易被氧化应激状态增强而吸引到大脑。这项研究突显了肠-脑串扰作为调节阿尔茨海默氏病神经变性的基本调节系统的重要性。新兴证据表明,肠菌群会影响大脑的免疫功能,并可能在神经系统疾病中发挥作用。在这里,作者提供了来自果蝇模型的体内证据,该模型支持肠道菌群在调节阿尔茨海默氏病进展中的作用。越来越多的证据表明,肠道菌群会影响大脑的免疫功能,并可能在神经系统疾病中起作用。在这里,作者提供了来自果蝇模型的体内证据,该模型支持肠道菌群在调节阿尔茨海默氏病进展中的作用。越来越多的证据表明,肠道菌群会影响大脑的免疫功能,并可能在神经系统疾病中起作用。在这里,作者提供了来自果蝇模型的体内证据,该模型支持肠道菌群在调节阿尔茨海默氏病进展中的作用。
更新日期:2017-06-26
中文翻译:

肠道微生物营养不良会加剧果蝇中阿尔茨海默氏病的进展。
Aβ42局部沉积引起的神经炎症在大脑中,阿尔茨海默氏病的发病机制和发展至关重要。然而,脑部炎症并不总是对局部原发性损伤的反应。肠道菌群失调最近已成为精神疾病的危险因素,它也可以引发脑部炎症反应。然而,肠营养不良是否也导致阿尔茨海默氏病仍不清楚。在这里我们表明,在果蝇阿尔茨海默氏病模型中,肠杆菌感染通过促进免疫血细胞募集到大脑而加剧了TNF-JNK介导的神经变性,从而加剧了阿尔茨海默氏病的进展。血细胞的遗传耗竭减弱了神经炎症并减轻了神经变性。我们进一步发现,肠感染会增加血细胞的运动能力,使它们更容易被氧化应激状态增强而吸引到大脑。这项研究突显了肠-脑串扰作为调节阿尔茨海默氏病神经变性的基本调节系统的重要性。新兴证据表明,肠菌群会影响大脑的免疫功能,并可能在神经系统疾病中发挥作用。在这里,作者提供了来自果蝇模型的体内证据,该模型支持肠道菌群在调节阿尔茨海默氏病进展中的作用。越来越多的证据表明,肠道菌群会影响大脑的免疫功能,并可能在神经系统疾病中起作用。在这里,作者提供了来自果蝇模型的体内证据,该模型支持肠道菌群在调节阿尔茨海默氏病进展中的作用。越来越多的证据表明,肠道菌群会影响大脑的免疫功能,并可能在神经系统疾病中起作用。在这里,作者提供了来自果蝇模型的体内证据,该模型支持肠道菌群在调节阿尔茨海默氏病进展中的作用。































 京公网安备 11010802027423号
京公网安备 11010802027423号