当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Analysis of Hydrogen-Bonding Effects on Excited-State Proton-Coupled Electron Transfer from a Series of Phenols to a Re(I) Polypyridyl Complex
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2017-05-01 00:00:00 , DOI: 10.1021/acs.jpcc.7b02449 Prateek Dongare 1 , Annabell G. Bonn 1 , Somnath Maji 1 , Leif Hammarström 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2017-05-01 00:00:00 , DOI: 10.1021/acs.jpcc.7b02449 Prateek Dongare 1 , Annabell G. Bonn 1 , Somnath Maji 1 , Leif Hammarström 1
Affiliation
|
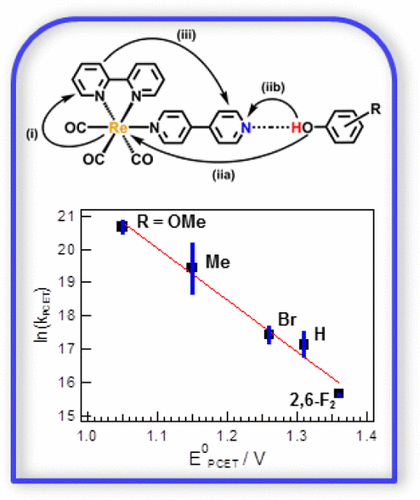
In the present study of proton-coupled electron transfer (PCET) reactions, the excited-state of a fac-[(CO)3ReI(bpy)(4,4′-bpy)]+ (bpy = 2,2′-bipyridine and 4,4′-bpy = 4,4′-bipyridine) complex was reductively quenched by a series of phenols. A variation of substituents on the phenols substantially alters their pKa and E° values and provides an opportunity to study photoinduced PCET as a function of their redox properties. Analyses of absorption spectral changes indicate that the phenols form a weak hydrogen bond with the pyridinic nitrogen of the 4,4′-bpy ligand in the ground-state, and ground-state association constant (KA) values were determined. This H-bonded adduct quenches the excited Re complex by PCET from the phenol, to form the reduced and protonated Re complex. The KA values obtained aid quantitative evaluation of the rate constant for the PCET reaction in the H-bonded adduct. Thus, photophysical studies and mechanistic analysis indicate that the reaction occurs via a concerted mechanistic pathway, for the unsubstituted phenol and phenols with electron-withdrawing substituents. Furthermore, the magnitude of the quenching varies systematically with the proton-coupled potentials of the phenols and not their hydrogen-bonding strength (as reflected in KA). This study is one of the first detailed analyses of intermolecular H-bonding between a self-assembling metal complex and a series of substituted phenols in an effort to study their relationship with the kinetic parameters in a photoinduced CPET reaction.
中文翻译:

氢键对激发态质子耦合电子从一系列苯酚到Re(I)聚吡啶配合物转移的键合效应分析
在目前的质子耦合电子转移(PCET)反应研究中,fac -[(CO)3 Re I(bpy)(4,4'-bpy)] +(bpy = 2,2' -联吡啶和4,4'-bpy = 4,4'-联吡啶)配合物通过一系列酚还原性淬灭。酚上取代基的变化大大改变了它们的p K a和E °值,并提供了根据其氧化还原特性研究光诱导PCET的机会。吸收光谱变化的分析表明,苯酚与基态的4,4'-bpy配体的吡啶吡啶氮形成弱氢键,且基态缔合常数(K A)的值已确定。这种氢键的加合物通过PCET从苯酚中淬灭了激发的Re络合物,形成了还原和质子化的Re络合物。获得的K A值有助于定量评估H键加合物中PCET反应的速率常数。因此,光物理研究和机理分析表明,对于未取代的苯酚和具有吸电子取代基的苯酚,反应是通过协调的机理途径发生的。此外,猝灭的幅度会随酚的质子偶合电位而不是其氢键强度而系统地变化(如K A中所反映的那样)。)。这项研究是对自组装金属配合物和一系列取代酚之间的分子间氢键的最早详细分析之一,目的是研究它们与光诱导CPET反应中动力学参数的关系。
更新日期:2017-06-02
中文翻译:

氢键对激发态质子耦合电子从一系列苯酚到Re(I)聚吡啶配合物转移的键合效应分析
在目前的质子耦合电子转移(PCET)反应研究中,fac -[(CO)3 Re I(bpy)(4,4'-bpy)] +(bpy = 2,2' -联吡啶和4,4'-bpy = 4,4'-联吡啶)配合物通过一系列酚还原性淬灭。酚上取代基的变化大大改变了它们的p K a和E °值,并提供了根据其氧化还原特性研究光诱导PCET的机会。吸收光谱变化的分析表明,苯酚与基态的4,4'-bpy配体的吡啶吡啶氮形成弱氢键,且基态缔合常数(K A)的值已确定。这种氢键的加合物通过PCET从苯酚中淬灭了激发的Re络合物,形成了还原和质子化的Re络合物。获得的K A值有助于定量评估H键加合物中PCET反应的速率常数。因此,光物理研究和机理分析表明,对于未取代的苯酚和具有吸电子取代基的苯酚,反应是通过协调的机理途径发生的。此外,猝灭的幅度会随酚的质子偶合电位而不是其氢键强度而系统地变化(如K A中所反映的那样)。)。这项研究是对自组装金属配合物和一系列取代酚之间的分子间氢键的最早详细分析之一,目的是研究它们与光诱导CPET反应中动力学参数的关系。











































 京公网安备 11010802027423号
京公网安备 11010802027423号