当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Diastereoselective Hydrolysis of Branched Malonate Diesters by Porcine Liver Esterase: Synthesis of 5-Benzyl-Substituted Cα-Methyl-β-proline and Catalytic Evaluation
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2017-05-31 15:21:00 , DOI: 10.1002/ejoc.201700605 Hari Kiran Kotapati 1 , Jamarii D. Robinson 1 , Daniel R. Lawrence 1 , Kimberly R. Fortner 1 , Caleb W. Stanford 1 , Douglas R. Powell 2 , Rainer Wardenga 3 , Uwe T. Bornscheuer 4 , Douglas S. Masterson 1
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2017-05-31 15:21:00 , DOI: 10.1002/ejoc.201700605 Hari Kiran Kotapati 1 , Jamarii D. Robinson 1 , Daniel R. Lawrence 1 , Kimberly R. Fortner 1 , Caleb W. Stanford 1 , Douglas R. Powell 2 , Rainer Wardenga 3 , Uwe T. Bornscheuer 4 , Douglas S. Masterson 1
Affiliation
|
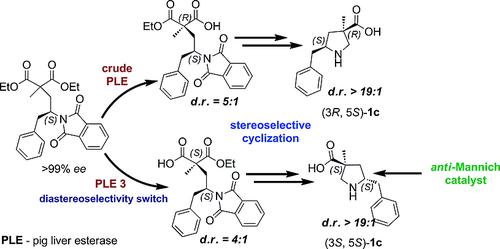
Malonate diesters with highly branched side chains containing a preexisting chiral center were prepared from optically pure amino alcohols and subjected to asymmetric enzymatic hydrolysis by Porcine Liver Esterase (PLE). Recombinant PLE isoenzymes have been utilized in this work to synthesize diastereomerically enriched malonate half-esters from enantiopure malonate diesters. The diastereomeric excess of the product half-esters was further improved in the later steps of synthesis either by simple recrystallization or flash column chromatography. The diastereomerically enriched half-ester was transformed into a novel 5-substituted Cα-methyl-β-proline analogue (3R,5S)-1c, in high optical purity employing a stereoselective cyclization methodology. This β-proline analogue was tested for activity as a catalyst of the Mannich reaction. The β-proline analogue derived from the hydrolysis reaction by the crude PLE appeared to catalyze the Mannich reaction between an α-imino ester and an aldehyde providing decent to good diastereoselectivities. However, the enantioselectivities in the reaction was low. The second diastereomer of the 5-benzyl-substituted Cα-methyl-β-proline, (3S,5S)-1c was prepared by enzymatic hydrolysis using PLE isoenzyme 3 and tested for its catalytic activity in the Mannich reaction. Amino acid, (3S,5S)-1c catalyzed the Mannich reaction between isovaleraldehyde and an α-imino ester yielding the “anti” selective product with an optical purity of 99 %ee.
中文翻译:

猪肝酯酶的非对映选择性水解分支丙二酸酯的研究:5-苄基取代的Cα-甲基-β-脯氨酸的合成及催化评价
由光学纯的氨基醇制备具有高度分支的侧链并含有预先存在的手性中心的丙二酸酯,并通过猪肝酯酶(PLE)进行不对称酶水解。重组PLE同工酶已用于这项工作中,从对映纯丙二酸二酯合成非对映异构富集的丙二酸半酯。通过简单的重结晶或快速柱色谱法,在合成的后续步骤中进一步改善了产物半酯的非对映异构体过量。非对映体的富集半酯被转化成一个新的5-取代的C α -甲基β -脯氨酸类似物(3 - [R,5小号) - 1C,以高光学纯度采用立体选择性环化方法。测试了该β-脯氨酸类似物作为曼尼希反应的催化剂的活性。粗PLE的水解反应所衍生的β-脯氨酸类似物似乎催化了α-亚氨基酯与醛之间的曼尼希反应,具有良好的非对映选择性。但是,反应中的对映选择性低。的5-苄基取代的C的第二非对映体α -甲基β -脯氨酸,(3小号,5小号) - 1C通过使用PLE同工酶3和用于在曼尼希反应的催化活性进行测试的酶水解制备。氨基酸(3 S,5 S)-1c催化异戊醛与α-亚氨基酯之间的曼尼希反应,得到光学纯度为99%ee的“反”选择性产物。
更新日期:2017-06-01
中文翻译:

猪肝酯酶的非对映选择性水解分支丙二酸酯的研究:5-苄基取代的Cα-甲基-β-脯氨酸的合成及催化评价
由光学纯的氨基醇制备具有高度分支的侧链并含有预先存在的手性中心的丙二酸酯,并通过猪肝酯酶(PLE)进行不对称酶水解。重组PLE同工酶已用于这项工作中,从对映纯丙二酸二酯合成非对映异构富集的丙二酸半酯。通过简单的重结晶或快速柱色谱法,在合成的后续步骤中进一步改善了产物半酯的非对映异构体过量。非对映体的富集半酯被转化成一个新的5-取代的C α -甲基β -脯氨酸类似物(3 - [R,5小号) - 1C,以高光学纯度采用立体选择性环化方法。测试了该β-脯氨酸类似物作为曼尼希反应的催化剂的活性。粗PLE的水解反应所衍生的β-脯氨酸类似物似乎催化了α-亚氨基酯与醛之间的曼尼希反应,具有良好的非对映选择性。但是,反应中的对映选择性低。的5-苄基取代的C的第二非对映体α -甲基β -脯氨酸,(3小号,5小号) - 1C通过使用PLE同工酶3和用于在曼尼希反应的催化活性进行测试的酶水解制备。氨基酸(3 S,5 S)-1c催化异戊醛与α-亚氨基酯之间的曼尼希反应,得到光学纯度为99%ee的“反”选择性产物。

































 京公网安备 11010802027423号
京公网安备 11010802027423号