当前位置:
X-MOL 学术
›
J. Control. Release
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chemotherapeutic drug-photothermal agent co-self-assembling nanoparticles for near-infrared fluorescence and photoacoustic dual-modal imaging-guided chemo-photothermal synergistic therapy
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2017-05-17 02:15:50 Yang Li, Guihua Liu, Jinyuan Ma, Jinyan Lin, Huirong Lin, Guanghao Su, Dengyue Chen, Shefang Ye, Xiaoyuan Chen, Xuan Zhu, Zhenqing Hou
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2017-05-17 02:15:50 Yang Li, Guihua Liu, Jinyuan Ma, Jinyan Lin, Huirong Lin, Guanghao Su, Dengyue Chen, Shefang Ye, Xiaoyuan Chen, Xuan Zhu, Zhenqing Hou
|
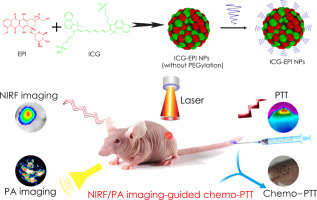
Multimodal imaging-guided synergistic combination therapy has shown great potential for cancer treatment. However, the nanocarrier-based theranostic systems suffer from batch-to-batch variation, complexity of multicomponent, poor drug loading, and carrier-related toxicity issues. To address these issues, herein we developed a novel carrier-free theranostic system with nanoscale characteristics for near-infrared fluorescence (NIRF) and photoacoustic (PA) dual-modal imaging-guided synergistic chemo-photothermal therapy (PTT). Indocyanine green (ICG) and epirubicin (EPI) could co-self-assemble into small molecular nanoparticles (NPs) in aqueous solution without any molecular precursor or excipient via collaborative interactions (electrostatic, π–π stacking, and hydrophobic interactions). The exceptionally high dual-drug loading (∼92wt%) ICG-EPI NPs showed good physiological stability, preferable photothermal response, excellent NIRF/PA imaging properties, pH-/photo-responsive drug release behavior, and promoted cellular endocytosis compared with free ICG or EPI. Importantly, the ICG-EPI NPs showed excellent tumor targeting ability with high spatial resolution and deep penetration via in vivo NIRF/PA dual-modal imaging. Moreover, in comparison with individual chemotherapy or PTT, the combinational chemo-PTT therapy of ICG-EPI NPs with NIR laser irradiation synergistically induced apoptosis and death of cancer cells in vitro, and showed synergistic chemo-PTT efficiency in vivo as evidenced by highly efficient tumor ablation. Furthermore, the ICG-EPI NPs exhibited inappreciable toxicity. This co-self-assembly of both FDA-approved agents provides a safe and “Molecular economical” strategy in the rational design of multifunctional nano-theranostic systems for real-time self-monitoring intracellular drug delivery and targeting multimodal imaging-guided synergistic combination therapy.
中文翻译:

用于近红外荧光和光声双模态成像引导化学光热协同治疗的化学药物-光热剂自组装纳米粒子
多模态成像引导的协同联合治疗已显示出巨大的癌症治疗潜力。然而,基于纳米载体的治疗方法系统存在批次间的差异,多组分的复杂性,不良的药物负载以及与载体相关的毒性问题。为了解决这些问题,本文中,我们开发了一种具有纳米级特征的新型无载体治疗疗法系统,用于近红外荧光(NIRF)和光声(PA)双模态成像引导的协同化学光热疗法(PTT)。吲哚菁绿(ICG)和表柔比星(EPI)可以通过协同相互作用(静电,π-π堆积和疏水相互作用)在水溶液中自组装成小分子纳米颗粒(NPs),而没有任何分子前体或赋形剂。与游离ICG相比,ICG-EPI NPs的超高双药载量(〜92wt%)显示出良好的生理稳定性,较好的光热响应,出色的NIRF / PA成像性能,pH- /光敏药物释放行为,并促进了细胞内吞作用或EPI。重要的是,通过体内NIRF / PA双模态成像,ICG-EPI NPs具有出色的肿瘤靶向能力,具有高空间分辨率和深度穿透力。此外,与单独的化学疗法或PTT相比,ICG-EPI NP的化学-PTT疗法与NIR激光照射的协同作用在体外协同诱导了癌细胞的凋亡和死亡,并在体内显示出协同的化学-PTT效率,这被高效证明了。肿瘤消融。此外,ICG-EPI NPs表现出不明显的毒性。
更新日期:2017-05-17
中文翻译:

用于近红外荧光和光声双模态成像引导化学光热协同治疗的化学药物-光热剂自组装纳米粒子
多模态成像引导的协同联合治疗已显示出巨大的癌症治疗潜力。然而,基于纳米载体的治疗方法系统存在批次间的差异,多组分的复杂性,不良的药物负载以及与载体相关的毒性问题。为了解决这些问题,本文中,我们开发了一种具有纳米级特征的新型无载体治疗疗法系统,用于近红外荧光(NIRF)和光声(PA)双模态成像引导的协同化学光热疗法(PTT)。吲哚菁绿(ICG)和表柔比星(EPI)可以通过协同相互作用(静电,π-π堆积和疏水相互作用)在水溶液中自组装成小分子纳米颗粒(NPs),而没有任何分子前体或赋形剂。与游离ICG相比,ICG-EPI NPs的超高双药载量(〜92wt%)显示出良好的生理稳定性,较好的光热响应,出色的NIRF / PA成像性能,pH- /光敏药物释放行为,并促进了细胞内吞作用或EPI。重要的是,通过体内NIRF / PA双模态成像,ICG-EPI NPs具有出色的肿瘤靶向能力,具有高空间分辨率和深度穿透力。此外,与单独的化学疗法或PTT相比,ICG-EPI NP的化学-PTT疗法与NIR激光照射的协同作用在体外协同诱导了癌细胞的凋亡和死亡,并在体内显示出协同的化学-PTT效率,这被高效证明了。肿瘤消融。此外,ICG-EPI NPs表现出不明显的毒性。

















































 京公网安备 11010802027423号
京公网安备 11010802027423号