当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Calcium Hydride Reactivity: Formation of an Anionic N‐Heterocyclic Olefin Ligand
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-05-05 , DOI: 10.1002/anie.201703037 Andrea Causero 1 , Holger Elsen 1 , Jürgen Pahl 1 , Sjoerd Harder 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-05-05 , DOI: 10.1002/anie.201703037 Andrea Causero 1 , Holger Elsen 1 , Jürgen Pahl 1 , Sjoerd Harder 1
Affiliation
|
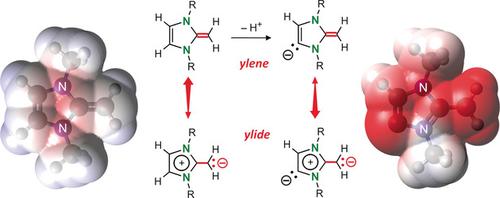
An anionic N‐heterocyclic olefin ligand was serendipitously obtained by reaction of an amidinate calcium hydride complex with 1,3‐dimethyl‐2‐methyleneimidazole (NHO). Instead of anticipated addition to the polarized C=CH2 bond to form an unstabilized alkylcalcium complex, deprotonation of the NHO ligand in the backbone was observed. Preference for deprotonation versus addition is explained by loss of aromaticity in the latter conversion. Theoretical calculations demonstrate the substantially increased ylidic character of this anionic NHO ligand which, like N‐heterocyclic dicarbenes, shows strong bifunctional coordination.
中文翻译:

氢化钙反应性:阴离子N-杂环烯烃配体的形成
通过an酰胺氢化钙配合物与1,3-二甲基-2-亚甲基咪唑(NHO)的反应偶然获得了一个阴离子N-杂环烯烃配体。观察到主链中NHO配体的去质子化,而不是预期的加到极化的C = CH 2键上以形成不稳定的烷基钙络合物。优先选择去质子化是通过在后者转化中芳族化合物的损失来解释的。理论计算表明,这种阴离子NHO配体的yidic特性大大提高,与N-杂环二碳烯一样,具有很强的双功能配位作用。
更新日期:2017-05-05
中文翻译:

氢化钙反应性:阴离子N-杂环烯烃配体的形成
通过an酰胺氢化钙配合物与1,3-二甲基-2-亚甲基咪唑(NHO)的反应偶然获得了一个阴离子N-杂环烯烃配体。观察到主链中NHO配体的去质子化,而不是预期的加到极化的C = CH 2键上以形成不稳定的烷基钙络合物。优先选择去质子化是通过在后者转化中芳族化合物的损失来解释的。理论计算表明,这种阴离子NHO配体的yidic特性大大提高,与N-杂环二碳烯一样,具有很强的双功能配位作用。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号