当前位置:
X-MOL 学术
›
Adv. Funct. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
CuF2 as Reversible Cathode for Fluoride Ion Batteries
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2017-05-04 , DOI: 10.1002/adfm.201701051 Duc Tho Thieu 1, 2 , Mohammed Hammad Fawey 1, 3 , Harshita Bhatia 1, 2 , Thomas Diemant 4 , Venkata Sai Kiran Chakravadhanula 1, 2, 5 , Rolf Jürgen Behm 2, 4 , Christian Kübel 1, 2, 5 , Maximilian Fichtner 1, 2
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2017-05-04 , DOI: 10.1002/adfm.201701051 Duc Tho Thieu 1, 2 , Mohammed Hammad Fawey 1, 3 , Harshita Bhatia 1, 2 , Thomas Diemant 4 , Venkata Sai Kiran Chakravadhanula 1, 2, 5 , Rolf Jürgen Behm 2, 4 , Christian Kübel 1, 2, 5 , Maximilian Fichtner 1, 2
Affiliation
|
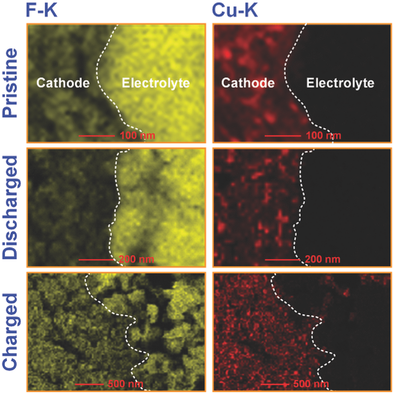
In the search for novel battery systems with high energy density and low cost, fluoride ion batteries have recently emerged as a further option to store electricity with very high volumetric energy densities. Among metal fluorides, CuF2 is an intriguing candidate for cathode materials due to its high specific capacity and high theoretical conversion potential. Here, the reversibility of CuF2 as a cathode material in the fluoride ion battery system employing a high F− conducting tysonite‐type La0.9Ba0.1F2.9 as an electrolyte and a metallic La as an anode is investigated. For the first time, the reversible conversion mechanism of CuF2 with the corresponding variation in fluorine content is reported on the basis of X‐ray photoelectron spectroscopy measurements and cathode/electrolyte interfacial studies by transmission electron microscopy. Investigation of the anode/electrolyte interface reveals structural variation upon cycling with the formation of intermediate layers consisting of i) hexagonal LaF3 and monoclinic La2O3 phases in the pristine interface; ii) two main phases of distorted orthorhombic LaF3 and monoclinic La2O3 after discharging; and iii) a tetragonal lanthanum oxyfluoride (LaOF) phase after charging. The fading mechanism of the cell capacity upon cycling can be explained by Cu diffusion into the electrolyte and side reactions due to the formation of the LaOF compound.
中文翻译:

CuF2作为氟离子电池的可逆阴极
在寻找具有高能量密度和低成本的新型电池系统时,氟化物离子电池近来已经成为存储具有很高体积能量密度的电的另一选择。在金属氟化物中,CuF 2由于其高的比容量和较高的理论转化率而成为阴极材料的诱人候选物。这里,CUF的可逆性2如在采用高F氟化物离子电池系统的阴极材料-导电tysonite型的La 0.9巴0.1 ˚F 2.9作为电解质和金属的La作为阳极进行了研究。CuF 2的可逆转换机制首次在X射线光电子能谱测量和透射电子显微镜对阴极/电解质界面的研究的基础上,报告了氟含量的相应变化。对阳极/电解质界面的研究表明,在循环过程中结构发生了变化,形成的中间层包括:i)原始界面中的六方LaF 3和单斜La 2 O 3相。ii)斜方晶LaF 3和单斜La 2 O 3的两个主要相放电后;iii)充电后为四方形的氟化氧镧(LaOF)相。循环时电池容量的衰减机理可以通过La扩散到电解质中以及由于LaOF化合物的形成引起的副反应来解释。
更新日期:2017-05-04
中文翻译:

CuF2作为氟离子电池的可逆阴极
在寻找具有高能量密度和低成本的新型电池系统时,氟化物离子电池近来已经成为存储具有很高体积能量密度的电的另一选择。在金属氟化物中,CuF 2由于其高的比容量和较高的理论转化率而成为阴极材料的诱人候选物。这里,CUF的可逆性2如在采用高F氟化物离子电池系统的阴极材料-导电tysonite型的La 0.9巴0.1 ˚F 2.9作为电解质和金属的La作为阳极进行了研究。CuF 2的可逆转换机制首次在X射线光电子能谱测量和透射电子显微镜对阴极/电解质界面的研究的基础上,报告了氟含量的相应变化。对阳极/电解质界面的研究表明,在循环过程中结构发生了变化,形成的中间层包括:i)原始界面中的六方LaF 3和单斜La 2 O 3相。ii)斜方晶LaF 3和单斜La 2 O 3的两个主要相放电后;iii)充电后为四方形的氟化氧镧(LaOF)相。循环时电池容量的衰减机理可以通过La扩散到电解质中以及由于LaOF化合物的形成引起的副反应来解释。































 京公网安备 11010802027423号
京公网安备 11010802027423号