Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rational molecular engineering of l-amino acid deaminase for production of α-ketoisovaleric acid from l-valine by Escherichia coli
RSC Advances ( IF 3.9 ) Pub Date : 2017-01-20 00:00:00 , DOI: 10.1039/c6ra26972a
Ruoxi Li 1, 2, 3, 4, 5 , Hossain Gazi Sakir 1, 2, 3, 4, 5 , Jianghua Li 1, 2, 3, 4, 5 , Hyun-dong Shin 6, 7, 8, 9 , Guocheng Du 1, 2, 3, 4, 5 , Jian Chen 1, 2, 3, 4, 5 , Long Liu 1, 2, 3, 4, 5
RSC Advances ( IF 3.9 ) Pub Date : 2017-01-20 00:00:00 , DOI: 10.1039/c6ra26972a
Ruoxi Li 1, 2, 3, 4, 5 , Hossain Gazi Sakir 1, 2, 3, 4, 5 , Jianghua Li 1, 2, 3, 4, 5 , Hyun-dong Shin 6, 7, 8, 9 , Guocheng Du 1, 2, 3, 4, 5 , Jian Chen 1, 2, 3, 4, 5 , Long Liu 1, 2, 3, 4, 5
Affiliation
|
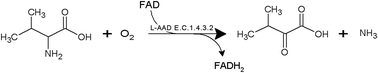
The targeted modification of enzymatic efficiency can drive an increased production of desired metabolites. α-Ketoisovaleric acid (KIV) is a candidate material for use in the pharmaceutical and food industries. In the present study, we aimed to enhance the biotransformation efficiency of L-amino acid deaminase (L-aad) from Proteus myxofaciens ATCC 19692 to improve the production of KIV from L-valine. First, L-aad was expressed in Escherichia coli BL21(DE3). We employed transformed E. coli cells as a whole-cell biocatalyst system and optimized their biochemical characteristics for the biotransformation of L-valine. Then, based on the known 3D structural model of L-aad from P. myxofaciens and the simulation results for docking with L-valine, four amino acid residues (N100, Q276, R316, and F318) were identified as potential target sites for mutagenesis. Next, we performed site-directed saturation mutagenesis to improve the biotransformation efficiency. With 11.3 g L−1 L-valine, the bioconversion efficiencies of a single-mutant strain (F318T) and a double-mutant strain (F318T and N100H) were 4.474 and 8.197 g L−1, respectively, whereas that of the wild-type strain was 2.014 g L−1 under optimal conditions. In summary, we developed a one-step process for KIV production via expressing P. myxofaciens L-aad in E. coli BL21(DE3) and enhanced the yield of KIV by site-directed saturation mutagenesis of L-aad.
中文翻译:

的合理的分子工程升-氨基酸脱氨酶生产α酮异戊酸的从升通过-缬氨酸大肠杆菌
酶促效率的靶向改变可以驱动所需代谢物的增加产量。α-酮异戊酸(KIV)是用于制药和食品工业的候选材料。在本研究中,我们旨在提高粘液变形杆菌ATCC 19692的L-氨基酸脱氨酶(L- aad)的生物转化效率,以提高L-缬氨酸生产KIV的效率。首先,L- aad在大肠杆菌BL21(DE3)中表达。我们将转化的大肠杆菌细胞用作全细胞生物催化剂系统,并针对L-的生物转化优化了它们的生化特性。缬氨酸。然后,基于已知的来自粘液假单胞菌的L -aad的3D结构模型和与L-缬氨酸对接的模拟结果,确定了四个氨基酸残基(N100,Q276,R316和F318)作为诱变的潜在目标位点。接下来,我们进行了定点饱和诱变,以提高生物转化效率。在11.3 g L -1 L缬氨酸下,单突变株(F318T)和双突变株(F318T和N100H)的生物转化效率分别为4.474和8.197 g L -1,而野生型在最佳条件下,典型菌株为2.014g L -1。总而言之,我们为KIV生产开发了一步法 通过在大肠杆菌BL21(DE3)中表达粘液腐单 胞菌L -aad进行表达,并通过L -aad的定点诱变提高了KIV的产量。
更新日期:2017-01-20
中文翻译:

的合理的分子工程升-氨基酸脱氨酶生产α酮异戊酸的从升通过-缬氨酸大肠杆菌
酶促效率的靶向改变可以驱动所需代谢物的增加产量。α-酮异戊酸(KIV)是用于制药和食品工业的候选材料。在本研究中,我们旨在提高粘液变形杆菌ATCC 19692的L-氨基酸脱氨酶(L- aad)的生物转化效率,以提高L-缬氨酸生产KIV的效率。首先,L- aad在大肠杆菌BL21(DE3)中表达。我们将转化的大肠杆菌细胞用作全细胞生物催化剂系统,并针对L-的生物转化优化了它们的生化特性。缬氨酸。然后,基于已知的来自粘液假单胞菌的L -aad的3D结构模型和与L-缬氨酸对接的模拟结果,确定了四个氨基酸残基(N100,Q276,R316和F318)作为诱变的潜在目标位点。接下来,我们进行了定点饱和诱变,以提高生物转化效率。在11.3 g L -1 L缬氨酸下,单突变株(F318T)和双突变株(F318T和N100H)的生物转化效率分别为4.474和8.197 g L -1,而野生型在最佳条件下,典型菌株为2.014g L -1。总而言之,我们为KIV生产开发了一步法 通过在大肠杆菌BL21(DE3)中表达粘液腐单 胞菌L -aad进行表达,并通过L -aad的定点诱变提高了KIV的产量。

































 京公网安备 11010802027423号
京公网安备 11010802027423号