当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Copper‐Catalyzed Ring‐Expansion/Thiolactonization via Azidation of Internal Olefinic C–H Bond under Mild Conditions
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2016-09-15 , DOI: 10.1002/adsc.201600675 Tenglong Guo 1 , Quanbin Jiang 1 , Zhengkun Yu 1, 2
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2016-09-15 , DOI: 10.1002/adsc.201600675 Tenglong Guo 1 , Quanbin Jiang 1 , Zhengkun Yu 1, 2
Affiliation
|
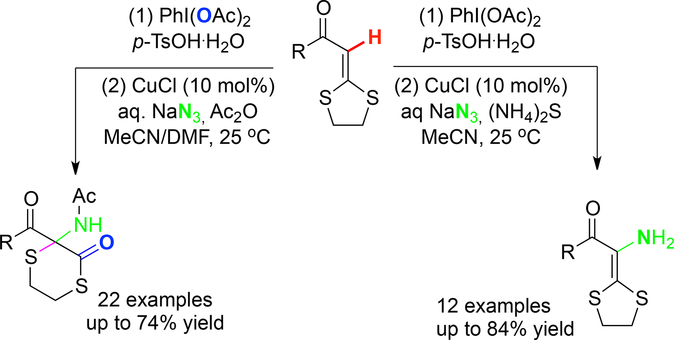
A copper(I)‐catalyzed, (diacetoxyiodo)benzene [PhI(OAc)2]‐mediated ring‐expansion/thiolactonization of α‐oxo ketene dithioacetals was efficiently realized via azidation of the internal olefinic C−H bond with sodium azide under mild conditions. Sequential amination, ring‐expansion rearrangement, and thiolactonization occurred to form aminated thiolactones in the presence of acetic anhydride as the additive, while only C−H amination to afford the unprotected enamines occurred when using ammonium sulfide as a reducing additive. The in situ generated vinyl azides were confirmed as the reactive intermediates, which were captured by phenylacetylene to produce triazoles. This protocol provides a concise route to thiolactone derivatives and unprotected enamines.
中文翻译:

在温和条件下通过内部烯烃C–H键的叠氮化进行铜催化的扩环/硫内酯化
通过在温和的条件下用叠氮化钠将内部C-H键与叠氮化钠叠氮化可以有效地实现铜(I)催化的(diacetoxyiodo)苯[PhI(OAc)2 ]介导的α-氧代乙烯酮二硫缩醛的扩环/硫内酯化反应条件。在使用乙酸酐作为添加剂的情况下,发生了顺序胺化,扩环重排和硫代内酯化反应,形成胺化的硫内酯,而当使用硫化铵作为还原性添加剂时,只有进行C-H胺化处理才能得到未保护的烯胺。该原位生成的乙烯基叠氮化物被证实为活性中间体,这是由苯乙炔捕获,以产生三唑。该方案为硫代内酯衍生物和未保护的烯胺提供了一条简明的途径。
更新日期:2016-09-15
中文翻译:

在温和条件下通过内部烯烃C–H键的叠氮化进行铜催化的扩环/硫内酯化
通过在温和的条件下用叠氮化钠将内部C-H键与叠氮化钠叠氮化可以有效地实现铜(I)催化的(diacetoxyiodo)苯[PhI(OAc)2 ]介导的α-氧代乙烯酮二硫缩醛的扩环/硫内酯化反应条件。在使用乙酸酐作为添加剂的情况下,发生了顺序胺化,扩环重排和硫代内酯化反应,形成胺化的硫内酯,而当使用硫化铵作为还原性添加剂时,只有进行C-H胺化处理才能得到未保护的烯胺。该原位生成的乙烯基叠氮化物被证实为活性中间体,这是由苯乙炔捕获,以产生三唑。该方案为硫代内酯衍生物和未保护的烯胺提供了一条简明的途径。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号