当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and Structure–Activity Relationships of a New Class of Oxadiazoles Targeting DprE1 as Antitubercular Agents
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2023-08-15 , DOI: 10.1021/acsmedchemlett.3c00295 Veena D Yadav 1 , Helena I Boshoff 1 , Lena Trifonov 1 , Jose Santinni O Roma 1 , Thomas R Ioerger 2 , Clifton E Barry 1 , Sangmi Oh 1
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2023-08-15 , DOI: 10.1021/acsmedchemlett.3c00295 Veena D Yadav 1 , Helena I Boshoff 1 , Lena Trifonov 1 , Jose Santinni O Roma 1 , Thomas R Ioerger 2 , Clifton E Barry 1 , Sangmi Oh 1
Affiliation
|
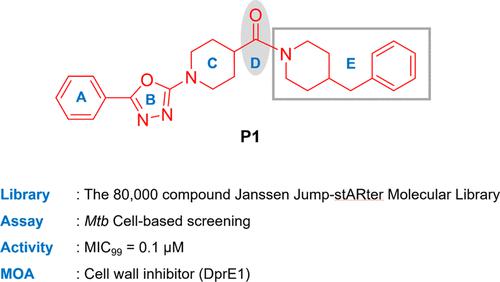
The continuing prevalence of drug-resistant tuberculosis threatens global TB control programs, highlighting the need to discover new drug candidates to feed the drug development pipeline. In this study, we describe a high-throughput screening hit (4-benzylpiperidin-1-yl)(1-(5-phenyl-1,3,4-oxadiazol-2-yl)piperidin-4-yl)methanone (P1) as a potent antitubercular agent. Structure–activity guided synthesis led to the discovery of several analogs with high in vitro potency. P1 was found to have promising potency against many drug-resistant strains, as well as drug-susceptible clinical isolates. It also showed cidality against Mtb growing in host macrophages. Whole genome sequencing of genomic DNA from resistant mutants raised to P1 revealed mutations in decaprenylphosphoryl-β-d-ribose 2′-oxidase (DprE1). This novel oxadiazole scaffold expands the set of chemical tools for targeting a well-validated pathway to treat tuberculosis.
中文翻译:

一类新型抗结核药物 DprE1 恶二唑的合成及其构效关系
耐药结核病的持续流行威胁着全球结核病控制计划,突出表明需要发现新的候选药物来满足药物开发渠道的需要。在这项研究中,我们描述了一个高通量筛选命中的 (4-benzylpiperidin-1-yl)(1-(5-苯基-1,3,4-oxadiazol-2-yl)piperidin-4-yl)methanone ( P1)作为有效的抗结核药。结构-活性引导合成导致了几种具有高体外效力的类似物的发现。人们发现P1对许多耐药菌株以及药物敏感的临床分离株具有良好的效力。它还显示出对宿主巨噬细胞中生长的结核分枝杆菌的杀灭作用。对P1 期耐药突变体的基因组 DNA 进行全基因组测序,发现十异戊二烯基磷酰基-β- d-核糖 2′-氧化酶 (DprE1) 发生突变。这种新型恶二唑支架扩展了化学工具集,用于针对经过充分验证的结核病治疗途径。
更新日期:2023-08-15
中文翻译:

一类新型抗结核药物 DprE1 恶二唑的合成及其构效关系
耐药结核病的持续流行威胁着全球结核病控制计划,突出表明需要发现新的候选药物来满足药物开发渠道的需要。在这项研究中,我们描述了一个高通量筛选命中的 (4-benzylpiperidin-1-yl)(1-(5-苯基-1,3,4-oxadiazol-2-yl)piperidin-4-yl)methanone ( P1)作为有效的抗结核药。结构-活性引导合成导致了几种具有高体外效力的类似物的发现。人们发现P1对许多耐药菌株以及药物敏感的临床分离株具有良好的效力。它还显示出对宿主巨噬细胞中生长的结核分枝杆菌的杀灭作用。对P1 期耐药突变体的基因组 DNA 进行全基因组测序,发现十异戊二烯基磷酰基-β- d-核糖 2′-氧化酶 (DprE1) 发生突变。这种新型恶二唑支架扩展了化学工具集,用于针对经过充分验证的结核病治疗途径。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号