当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
N-(5-Amino-9H-benzo[a]phenoxazin-9-ylidene)propan-1-aminium chlorides as antifungal agents and NIR fluorescent probes
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2021-3-26 , DOI: 10.1039/d1nj00879j Rui P. C. L. Sousa 1, 2, 3, 4, 5 , João C. C. Ferreira 1, 2, 3, 4, 5 , Maria João Sousa 3, 4, 5, 6, 7 , M. Sameiro T. Gonçalves 1, 2, 3, 4, 5
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2021-3-26 , DOI: 10.1039/d1nj00879j Rui P. C. L. Sousa 1, 2, 3, 4, 5 , João C. C. Ferreira 1, 2, 3, 4, 5 , Maria João Sousa 3, 4, 5, 6, 7 , M. Sameiro T. Gonçalves 1, 2, 3, 4, 5
Affiliation
|
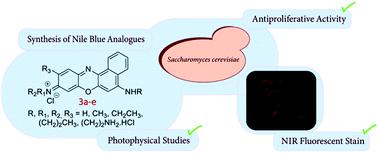
The search for benzo[a]phenoxazines, Nile Blue derivatives, with high antifungal activity and cell labelling capacity based on our previously published work on this type of compounds led us to the design of compounds with specific substituents in the polycyclic system. Thus, in the present work, four new benzo[a]phenoxazinium chlorides, possessing at the 5-position amino or (3-aminopropyl) amino groups and at the 9-position propylamino or dipropylamino groups, were synthesized. Another analogue, with a (3-aminopropyl) amino group at the 5-position, an ethyl amino group at the 9-position and a methyl group at the 10-position of the polycyclic system, was also synthesized for comparison in the studies performed. The fundamental photophysics (absorption and fluorescence emission) was studied in absolute ethanol, water, and other aqueous solutions of different pH values, relevant for the potential biological applications of these compounds. The antiproliferative activity of the synthesized benzo[a]phenoxazinium chlorides was determined using Saccharomyces cerevisiae PYCC 4072 and the microdilution method described for antifungal susceptibility tests in yeast. All compounds revealed antifungal activity, the most active being the one possessing an amino group at the 5-position and an aminopropyl group at the 9-position. The potential as fluorescent probes was evaluated by fluorescence microscopy, using S. cerevisae as a model system of eukaryotic cells, and it was found that the benzo[a]phenoxazinium chlorides stained the cells with preferential accumulation that seems to appear at the vacuolar membrane and/or the perinuclear membrane of the endoplasmatic reticulum.
中文翻译:

N-(5-氨基-9H-苯并[a]苯恶嗪-9-亚丙基)丙-1-铵氯化物作为抗真菌剂和NIR荧光探针
基于我们先前发表的关于这类化合物的研究,对具有高抗真菌活性和细胞标记能力的苯并[ a ]吩恶嗪,尼罗河蓝衍生物的探索促使我们设计了在多环系统中具有特定取代基的化合物。因此,在目前的工作中,有四个新的苯并[ a]合成了具有5-位氨基或(3-氨基丙基)氨基和9位丙基氨基或二丙基氨基的]吩恶嗪氯化物。还合成了另一种类似物,在多环系统的5位带有(3-氨基丙基)氨基,在9位带有乙基氨基,在10位带有甲基。 。在无水乙醇,水和其他pH值不同的水溶液中研究了基本的光物理性质(吸收和荧光发射),这与这些化合物的潜在生物应用有关。使用酿酒酵母测定了合成的苯并[ a ]苯恶嗪氯化物的抗增殖活性PYCC 4072和微量稀释方法用于酵母中的抗真菌药敏试验。所有化合物均显示出抗真菌活性,其中最活跃的是在5位具有氨基,在9位具有氨基丙基的化合物。使用酿酒酵母作为真核细胞的模型系统,通过荧光显微镜评估了作为荧光探针的潜力,发现苯并[ a ]苯恶嗪氯化物使细胞出现了优先积累,这种积累似乎出现在液泡膜和/或内质网的核周膜。
更新日期:2021-04-15
中文翻译:

N-(5-氨基-9H-苯并[a]苯恶嗪-9-亚丙基)丙-1-铵氯化物作为抗真菌剂和NIR荧光探针
基于我们先前发表的关于这类化合物的研究,对具有高抗真菌活性和细胞标记能力的苯并[ a ]吩恶嗪,尼罗河蓝衍生物的探索促使我们设计了在多环系统中具有特定取代基的化合物。因此,在目前的工作中,有四个新的苯并[ a]合成了具有5-位氨基或(3-氨基丙基)氨基和9位丙基氨基或二丙基氨基的]吩恶嗪氯化物。还合成了另一种类似物,在多环系统的5位带有(3-氨基丙基)氨基,在9位带有乙基氨基,在10位带有甲基。 。在无水乙醇,水和其他pH值不同的水溶液中研究了基本的光物理性质(吸收和荧光发射),这与这些化合物的潜在生物应用有关。使用酿酒酵母测定了合成的苯并[ a ]苯恶嗪氯化物的抗增殖活性PYCC 4072和微量稀释方法用于酵母中的抗真菌药敏试验。所有化合物均显示出抗真菌活性,其中最活跃的是在5位具有氨基,在9位具有氨基丙基的化合物。使用酿酒酵母作为真核细胞的模型系统,通过荧光显微镜评估了作为荧光探针的潜力,发现苯并[ a ]苯恶嗪氯化物使细胞出现了优先积累,这种积累似乎出现在液泡膜和/或内质网的核周膜。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号