Topic:Frozen reinforced microneedles loaded with NIR-photothermal nanozyme
for keratitis treatment.
Original link: https://doi.org/10.1016/j.nantod.2023.102000
Journal: Nano Today
IF: 17.4
Reporter: Ximei Han, Master of Grade 2022

Developing a potential alternative with safe, minimally invasive, and highly effective antimicrobial properties is significant for treating bacterial keratitis. In this paper, we present novel nanozyme-loaded frozen reinforced microneedles (FR-MNs) with the ability to deliver the nanozyme controllably inside the cornea to treat keratitis. The nanozymes constructed by the iron ion, tannic acid (TA), and poly (vinylpyrrolidone; PVP) (FeTAP) are incorporated within the MNs tips. The freezing process could greatly enhance the mechanical strength of the tips. Taking advantage of the pH-dependent peroxidase-mimetic activities of FeTAP, the FeTAP integrated MNs can catalyze hydrogen peroxide (H2O2) to produce much more oxidative hydroxyl (•OH) in acidic condition to kill bacteria. Meanwhile, the excellent photothermal efficiency of FeTAP can further enhance the ability of bacterial killing under near-infrared (NIR) irradiation. In addition, the photothermal action can rapidly increase the temperature of the frozen microneedles and subsequently soften the MNs after penetration, which can result in better adaptation of MNs to soft tissues. Based on the nanozyme-loaded FR-MNs, we have demonstrated that this formulation exhibited superior therapeutic potential in the rat eye infection model compared with conventional eye drops. These features impart the proposed nanozyme-loaded FR-MNs with great scientific value and promising clinical applications.

Bacterial keratitis, also known as bacterial corneal ulcers, is a kind of corneal inflammation caused by bacterial infections, such as Staphylococcus, Streptococcus, Streptococcus, and Pseudomonas. The improper or ineffective treatment of bacterial keratitis may lead to corneal perforations, intraocular infections, and eventually irreversible visual impairment and blindness. Antibiotic eye drops are the most widely used therapy for treating bacterial keratitis. However, due to the presence of the distinctive anatomical and physiological, structural characteristics of the cornea, such as the tight junction in the epithelium, the drug within the eye drop will be released rapidly after topical administration, with a short resistance time and poor bioavailability. In addition, the misuse of antibiotics has resulted in the emergence of antibiotic-resistant bacteria, overshadowing the future of antibiotic-based treatment. In contrast, intraocular injection based on a traditional hypodermic needle can penetrate the surface barrier and improve topical delivery with prolonged drug resistance, while patient compliance is poor because of the pain, frequent visits requirement, and risk of infection and bleeding. Thus, ocular delivery of localized, persistent, and highly effective antimicrobial agents, as well as favorable patient compliance, is highly anticipated for treating bacterial keratitis.
Herein, we present novel frozen reinforced microneedles (FR-MNs) loaded with near-infrared (NIR)-photothermal nanozyme for keratitis treatment, as schemed in Fig. 1. Nanozymes denote nanomaterials with mimetic enzyme activity. Because of the ease of preparation, regulated catalytic activity, high stability, cost-effectiveness, and facile handling, nanozymes have become promising alternatives to natural enzymes in various applications. Specifically, the nanozymes assembled by iron ion and polyhydric phenol have exhibited excellent biocompatibility, biodegradability, pH-dependent catalytic activity, and reactive oxygen species (ROS) formation or scavenging abilities. However, effectively and locally delivering these nanozymes to the ocular surface remains a challenge. Alternatively, microneedles (MNs) are an array of tiny needles with a micron scale, which have been widely used to penetrate the epidermal for painless, non-invasive, and highly efficient transdermal drug delivery. Drugs, functional cytokines, stem cells, proteins, two-dimensional materials, and metal-organic framework materials have been incorporated within MNs for various therapeutic purposes. Nevertheless, some MNs systems from natural polymers are too soft to attain enough tissue-piercing strength. Thus, how to enhance the mechanical strength to ensure that the MNs can penetrate tissues and at the same time achieve adaptive softening after penetration is a difficulty for MNs when applied in soft tissues.

Fig.1. The schematic diagram shows the composition of the FR-MNs and their application for treating keratitis.

1. Fabrication and characterization of FR-MNs.
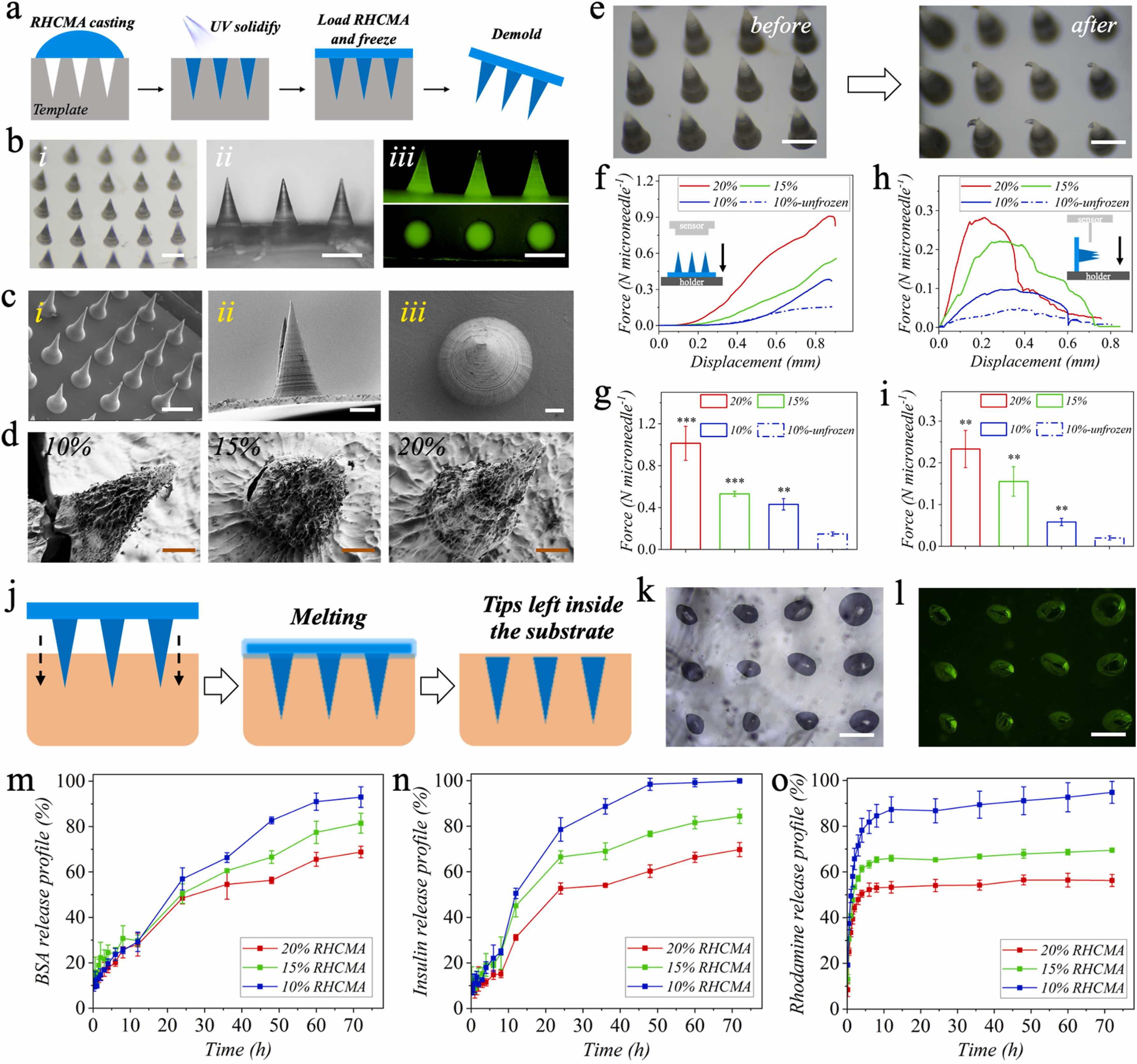
Fig.2. Fabrication and characterization of FR-MNs. a) Schematic diagram of the FR-MNs fabrication process. b) Digital (i, ii) and fluorescent (iii) images of the FR-MNs. c) SEM images of the FR-MNs with different side views. d) SEM images of the freeze-drying FR-MNs fabricated with different RHCMA concentrations. e) Digital images of the MNs before and after compression. f, g) Compressive curves and forces of different FR-MNs and unfrozen MNs. h, i) Shear curves and forces of different FR-MNs and unfrozen MNs. (n = 3, biologically independent samples). Data are expressed as mean ± SD. j) Schematic diagram of the penetration process of FR-MNs into the substrate. k) Digital and l) fluorescent images of the agarose after being penetrated with the MNs. Releasing profiles of m) BSA, n) insulin, and o) RhB within different FR-MNs. Scale bars: 500 μm in (b), 500 μm, 100 μm, and 30 μm in (c, i), ii), and iii)), 50 μm in (d), 500 μm in (e), and 200 μm in (k) and (l).
2. Characterization of FeTAP nanozyme.
.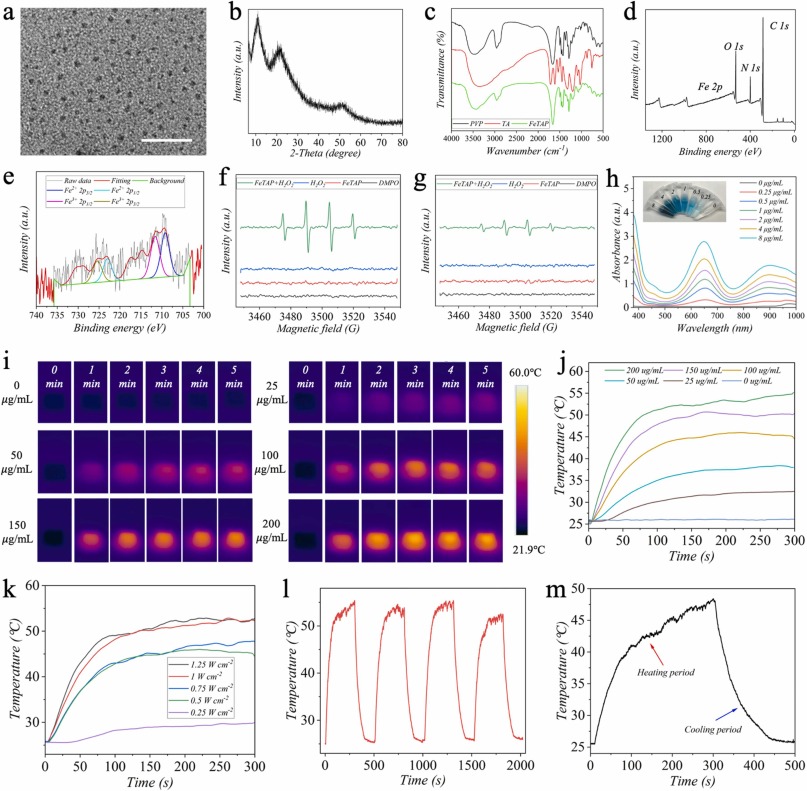
Fig.3. Characterization of FeTAP nanozyme. a) TEM image of FeTAP. b) XRD pattern of FeTAP. c) FTIR spectrum of PVP, TA, and FeTAP. d) XPS spectrum of FeTAP. e) Fe 2p XPS spectrum of FeTAP. ESR spectra of FeTAP, H2O2, and FeTAP in the presence of H2O2 under f) acidic situation (pH=5.5) and g) neutral situation (pH=7.4). h) Absorbance curves of TMBox after incubating with varying concentrations of FeTAP. The inset image is the corresponding color change. Photothermal images i) and heating curves j) of MNs incorporated with FeTAP. k) Heating curves of MNs incorporated with FeTAP under different irradiation power densities. l) Photothermal stability of TeTAP. m) Temperature variation of primary heating and cooling of FeTAP. Scale bar: 20 nm in (a).
3. Antibacterial effect of FeTAP in vitro.

Fig.4. Antibacterial effect of FeTAP in vitro. a) Agar plate photographs of bacteria after treatment with buffer (control), H2O2, FeTAP, and FeTAP+H2O2, with or without NIR irradiation. b) SEM images of bacteria after the same treatment as in (a). Fluorescent Live/Dead images of c) S. aureus and d) E. coli after the same treatment as in (a). e) Viability of S. aureus. f) Viability of E. coli. Scale bars: 1 μm for S.aureus in (b), 2 μm for E.coli in (b), 50 μm in (c, d). * p < 0.05, ** p < 0.01, *** p < 0.001, compared in the groups; ### p < 0.001, compared between the groups.
4. Influence of FeTAP-loaded MNs on corneal cells.

Fig.5. Influence of FeTAP-loaded MNs on corneal cells. a) The Live/Dead staining images of HKs and HCECs after culturing in the control, MNs, and MNs with different concentrations of FeTAP for 3 days. The cell viability of b) HCECs and c) HKs after culturing for 1, 2, and 3 days. The immunofluorescent staining of d) ALDH3A1, and e) CK12 in HKs and HCECs, respectively. Scale bars: 200 μm in (a), 100 μm in (d, e).
5. In vivo antibacterial evaluation of FeTAP-loaded FR-MNs.

Fig.6. In vivo antibacterial evaluation of FeTAP-loaded FR-MNs. a) Representative photographs of infected corneas treated with drops, FR-MNs, FeTAP + FR-MNs, and FeTAP + FR-MNs + NIR for different times. b) Agar plate photographs of bacteria derived from the infected cornea after the same treatment in (a). c) Histogram of the bacterial number on day 1 and day 7. d) Representative HE staining images of the infected cornea after the same treatment in (a) for 7 days. e) Corneal epithelial and stromal thickness of the infected cornea after the same treatment in (a) for 7 days. Scale bars: 1.5 mm in (a), 100 μm in (d), 50 μm in the
enlarged images.
6. Immunological staining to verify the antibacterial evaluation of FeTAP-loaded FR-MNs.

Fig.7. Immunological staining to verify the antibacterial evaluation of FeTAP-loaded FR-MNs. Representative immunofluorescent a) TNF-α, b) IL-6 and immunohistochemistry c) CD68, d) VEGF staining images of infected corneas after treated with drops, FR-MNs, FeTAP + FR-MNs, and FeTAP + FR-MNs + NIR for 7 days. Histogram of e) TNF-α positive cells, f) IL-6 positive cells, g) CD68 MFI, and h) VEGF MFI. Scale bars: 100 μm in (a-d), 50 μm in the enlarged images. * p < 0.05, **p < 0.01, *** p < 0.001.

In summary, we present nanozyme-loaded frozen reinforced microneedles with the ability to deliver the nanozyme controllably inside the cornea to treat keratitis. The freezing process could significantly enhance the mechanical strength, and controllable release of molecules with different molecular weights was observed in the FR-MNs. FeTAP could catalyze H2O2 to produce much more •OH at the acidic condition to kill bacteria. Meanwhile, the excellent photothermal efficiency of FeTAP can further enhance the ability of bacterial killing under NIR irradiation. In addition, the FeTAP-loaded MNs exhibited excellent biocompatibility to HKs and HCECs. Notably, it was demonstrated that this formulation exhibited superior therapeutic potential in the rat eye infection model compared with conventional eye drops. These features impart the nanozyme-loaded FR-MNs with great scientific value and promising clinical applications.
