Before NJU
1. Angew. Chem. Int. Ed. 2023. Late-Stage Aryl C-H Bond Cyclopropenylation with Cyclopropenium Cations. Tu, H.-F.;Jeandin, A.; Bon, C.; Lima, F.; Brocklehurst, C.; Suero, M. G. Among the most accessed articles in July 2023[https://doi.org/10.1002/anie.202308379]

2. J. Am. Chem. Soc. 2022, 144, 16737–16743. Catalytic Synthesis of Cyclopropenium Cations with Rh-carbynoids. Tu, H.-F.; Jeandin, A.; Suero, M. G. Among the most read articles in September 2022[https://doi.org/10.1021/jacs.2c07769]

3. Adv. Synth. Catal. 2022, 364, 3432-3437. Iridium-Catalyzed Intermolecular Asymmetric Allylic Amination with Pyridones. Tu, H.-F.; Nie, Y.-H.; Zheng, C.; You, S.-L.[https://doi.org/10.1002/adsc.202200347]

4. Nat. Chem. 2020, 12, 838–844. Time-Dependent Enantiodivergent Synthesis via Sequential Kinetic Resolution. Tu, H.-F.; Yang, P.; Lin, Z.-H.; Zheng, C.; You, S.-L.(入选2020年度C&EN三项轰动性合成工作之一,Highlighted byphys.org. ,Chemistry World , X-molandChem.)[ https://doi.org/10.1038/s41557-020-0489-1]
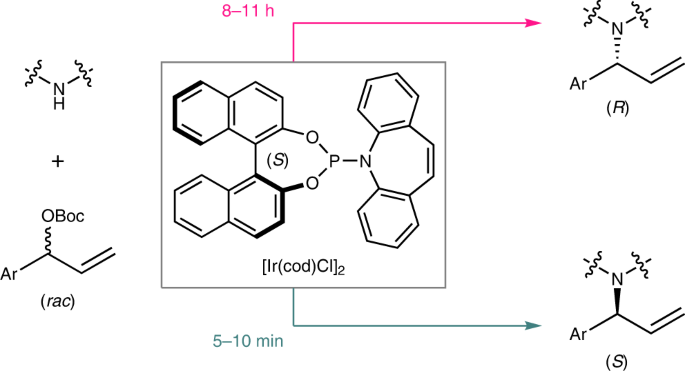
5. Chem. Rev. 2019, 119, 1855–1969. Iridium-Catalyzed Asymmetric Allylic Substitution Reactions. Cheng, Q.;#Tu, H.-F.;# Zheng, C.; Qu, J.-P.; Helmchen, G.; You, S.-L. (#: Co-first author.)[https://doi.org/10.1021/acs.chemrev.8b00506]

6. Nat. Catal. 2018, 1, 601–608. Enantioselective Dearomative Prenylation of Indole Derivatives.Tu, H.-F.;# Zhang, X.;# Zheng, C.; Zhu, M.; You, S.-L. (#: Co-first author, Highlighted byChem., Chemistry News and CBG News.)[https://doi.org/10.1038/s41929-018-0111-8]

7. Angew. Chem. Int. Ed. 2017, 56, 3237-3241. Iridium-Catalyzed Intermolecular Asymmetric Dearomatization of β-Naphthols with Allyl Alcohols or Allyl Ethers.Tu, H.-F.; Zheng, C.; Xu, R.-Q.; Liu, X.-J.; You, S.-L. (VIP, Highlighted by Synfacts and X-mol.)[ https://doi.org/10.1002/anie.201609654]

8. Org. Lett. 2019, 21, 6130-6134. Rhodium-Catalyzed Asymmetric Allylic Dearomatization of β-Naphthols: Enantioselective Control of Prochiral Nucleophiles. Tang, S.-B.; Tu, H.-F.; Zhang, X.; You, S.-L.[Link]
9. J. Am. Chem. Soc. 2018, 140, 7737-7742. Regio- and Enantioselective Rhodium-Catalyzed Allylic Alkylation of Racemic Allylic Alcohols with 1,3-Diketones. Tang, S.-B.; Zhang, X.; Tu, H.-F.; You, S.-L.[Link]
10. Org. Biomol. Chem. 2018, 16, 8700–8703. Rh-Catalyzed Aminative Dearomatization of 2-Naphthols. Yi, J.-C.; Tu, H.-F.; You, S.-L.[Link]
11. Angew. Chem. Int. Ed. 2016, 55, 15137-15141. Palladium(0)-Catalyzed Intermolecular Arylative Dearomatization of β-Naphthols. Xu, R.-Q.; Yang, P.; Tu, H.-F.; Wang, S.-G.; You, S.-L.[Link]
12. Chem. Sci. 2015, 6, 4525-4529. Ligand-Enabled Ir-Catalyzed Intermolecular Diastereoselective and Enantioselective Allylic Alkylation of 3-Substituted Indoles. Zhang, X.; Liu, W.-B.;Tu, H.-F.; You, S.-L.[Link]
13. Chem. Eur. J. 2014, 20, 2445–2448. One-Pot Synthesis of Fused Pyrroles through a Key Gold-Catalysis-Triggered Cascade. Zheng, Z.; Tu, H.-F.; Zhang, L.[Link]
14. Chem. Commun. 2013, 49, 701–703. Highly Regioselective Synthesis of Fused Seven-Membered Rings through Copper-Catalyzed Cross-Coupling. Sang, P.; Yu, M.; Tu, H.-F.; Zou, J.; Zhang, Y.[Link]