一:基于氮杂螺环骨架,开发出了一类新的双齿氮配体吡咯烷-噁唑啉(SPDO), 并发展了Cu/SPDO催化体系,1)通过氧化偶联实现了轴手性BINOL,NOBIN及3-苯基吲哚化合物的制备;2)其次通过[3+2]环加成实现了苯并呋喃的结构构建及海洋天然产物Corsifuran A 和B的合成。
1. Copper-Complex-Catalyzed Asymmetric Aerobic Oxidative Cross-Coupling of 2-Naphthols: Enantioselective Synthesis of 3,3′-Substituted C1-Symmetric BINOLs. Angew. chem. Int. Ed. 2019, 58, 11023. https://onlinelibrary.wiley.com/doi/full/10.1002/anie.201903435
Highlight by X-Mol: Angew. Chem.:手性Cu/SPDO配合物催化的氧化交叉偶联反应,高效构建3,3'-双取代BINOLs- X-MOL资讯

2. Enantioselective Synthesis of 3,3′-Disubstituted 2-Amino-2′-hydroxy-1,1′-binaphthyls by Copper-Catalyzed Aerobic Oxidative Cross-Coupling. Angew. chem. Int. Ed. 2021, 60, 7061. https://onlinelibrary.wiley.com/doi/full/10.1002/anie.202015001
Highlight by 科学网: 铜催化的好氧氧化交叉偶联反应研究取得新突破—小柯机器人—科学网 (sciencenet.cn)
Highlight by 纳米人-Angew:Cu(I)/SPDO催化萘胺/萘酚空气室温氧化偶联合成手性螺环产物 (nanoer.net)
Highlight by 铜催化对映选择性合成3.3-二取代的2-氨基-2‘-羟基1,1’-联萘 – 化学慧 (chemhui.com)
Highlight by 《德国应化》发表上海交通大学涂永强院士团队的手性Cu/SPDO配合物催化的氧化交叉偶联反应,高效构建3,3'-双取代NOBINS-变革性分子前沿科学中心 (sjtu.edu.cn)

3. Atroposelective Synthesis of Axially Chiral 3-Arylindoles by Copper-Catalyzed Asymmetric Cross-Coupling of Indoles with Quinones and Naphthoquinones. Org. Lett. 2020, 22, 4995. https://pubs.acs.org/doi/abs/10.1021/acs.orglett.0c01558

4.Enantioselective Construction of 2-Aryl-2,3-dihydrobenzofuran Scaffolds Using Cu/SPDO-Catalyzed [3 + 2] Cycloaddition. Org. Lett. 2021, 23, 1258 https://pubs.acs.org/doi/abs/10.1021/acs.orglett.0c04241

二:基于氮杂螺环骨架,开发出了一类新的吡咯烷型胺类催化剂, 并发展了基于Michael反应的系列反应,并将其用于天然产物及药物分子合成。
5. A Spiro-Pyrrolidine Organocatalyst and Its Application to Catalytic Asymmetric Michael Addition for the Construction of All-Carbon Quaternary Centers. Chem. Commun., 2015,51, 9979. https://pubs.rsc.org/en/content/articlelanding/2015/CC/C5CC02765A

6. Catalytic Asymmetric Cascade Using Spiro-Pyrrolidine Organocatalyst: Efficient Construction of Hydrophenanthridine Derivatives. Org. Lett. 2017, 19, 6618. https://pubs.acs.org/doi/abs/10.1021/acs.orglett.7b03330

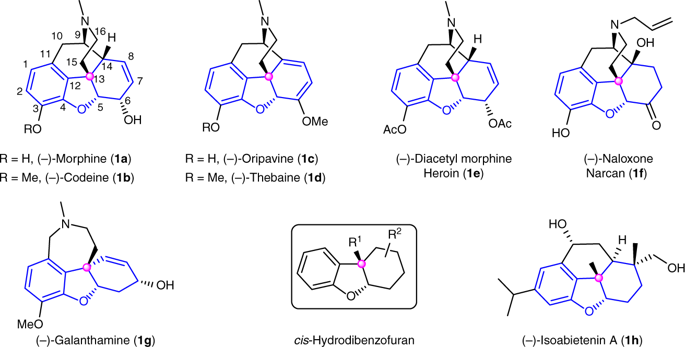
7 Enantioselective synthesis of cis-hydrobenzofurans bearing all-carbon quaternary stereocenters and application to total synthesis of (‒)-morphine. Nat Commun 2019, 10, 2507 https://www.nature.com/articles/s41467-019-10398-4?utm_source=xmol&utm_medium=affiliate&utm_content=meta&utm_campaign=DDCN_1_GL01_metadata
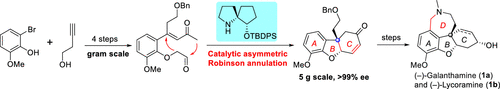
8. Catalytic Asymmetric Total Syntheses of (-)-Galanthamine and (-)-Lycoramine. J. Org. Chem. 2019, 84, 12664. https://pubs.acs.org/doi/abs/10.1021/acs.joc.9b01971
.9. Asymmetric intramolecular Friedel–Crafts reaction catalyzed by a spiropyrrolidine organocatalyst: Enantioselective construction of indolizine and azepine frameworks. Tetrahedron Lett. 2018, 59, 4015. https://www.sciencedirect.com/science/article/pii/S0040403918311651

三:基于Semipinacol重排反应为关键步骤,合成了3-epi-tenuipesine A合成。
10. A Synthetic Approach for Constructing the 3/6/6/5-Fused Tetracyclic Skeleton of Tenuipesine A. Chem. Asian, J. 2014, 9, 724. https://onlinelibrary.wiley.com/doi/abs/10.1002/asia.201301581

