仅列举了近8年的部分论文
01. Gold‐Catalyzed 1,2‐Acyloxy Migration/Intramolecular [3+2] 1,3‐Dipolar Cycloaddtion Cascade Reaction: An Efficient Strategy for Syntheses of Medium‐Sized‐Ring Ethers and Amines
Angewandte Chemie International Edition ( IF 16.823 ) Pub Date : 2014-08-11 , DOI: 10.1002/anie.201406486
Changgui Zhao 1 , Xingang Xie 1 , Shuangshuang Duan 1 , Huilin Li 1 , Ran Fang 1 , Xuegong She 1, 2

一种基于金催化的级联反应形成中型环醚和胺的高效策略,该过程涉及烯丙基酯异构化和分子内[3 + 2]环化。通过这种转化获得了各种多取代的中环不饱和醚和胺。该方法实现了中等规模的环合成中相对较少的过渡金属催化的分子内环加成反应。
02. Biomimetic Total Syntheses of (+)-Dihydrolyfoline and (−)-5-epi-Dihydrolyfoline
Organic Letters ( IF 6.072 ) Pub Date : 2015-04-17 00:00:00 , DOI: 10.1021/acs.orglett.5b00846
Ruoming Mei 1 , Dengyu Xu 1 , Haitao Hu 1 , Dengpeng Song 1 , Hao Zhang 1 , Donghui Ma 1 , Xingang Xie 1 , Xuegong She 1, 2

(+)-二氢脯氨酸和(-)-5-表-二氢脯氨酸的第一批不对称总合成分别通过五步和六步完成,总收率分别为4.6%和14%,其中手性联芳基轴是通过苯酚的生物遗传酶氧化的偶联反应实现高度区域选择性和立体选择性,并通过酶促曼尼希反应制备了所需的喹喔啉酮核心骨架。
03. Short and Scalable Total Synthesis of Myrioneuron Alkaloids (±)-α,β-Myrifabral A and B
Organic Letters ( IF 6.072 ) Pub Date : 2016-02-05 00:00:00 , DOI: 10.1021/acs.orglett.6b00005
Dengpeng Song 1 , Zhengshen Wang 1 , Ruoming Mei 1 , Weiwei Zhang 1 , Donghui Ma 1 , Dengyu Xu 1 , Xingang Xie 1 , Xuegong She 1, 2

Myrioneuron生物碱(±)-α,β-myrifabralA和B的第一个全合成反应仅需四个步骤。这种简短的合成关键的是串联曼尼希/酰胺化反应来快速构建核心框架和两个碳立体中心。该合成路线可大规模制备这些抗丙型肝炎病毒(HCV)的天然产物。
04. Bioinspired Collective Syntheses of Iboga-Type Indole Alkaloids
Organic Letters ( IF 6.072 ) Pub Date : 2016-05-10 00:00:00 , DOI: 10.1021/acs.orglett.6b00989
Gaoyuan Zhao 1 , Xingang Xie 1 , Haiyu Sun 1 , Ziyun Yuan 1 , Zhuliang Zhong 1 , Shouchu Tang 1 , Xuegong She 1, 2

一种生物启发性的集群合成策略在七个iboga型吲哚生物碱的总合成中的应用:(±)-他汀丁,(±)-ibogamine,(±)-ibogaine,(±)-ibogaine羟基吲哚肾上腺素,(±) -3-氧代bogaine羟吲哚胺,(±)-iboluteine和(±)-ervaoffinesD。他培他汀及其同类物可作为iboga前体,用于随后的仿生转化为其他iboga型生物碱。
05. Protecting-Group-Free Total Synthesis of (−)-Lycopodine via Phosphoric Acid Promoted Alkyne Aza-Prins Cyclization
Organic Letters ( IF 6.072 ) Pub Date : 2016-08-16 00:00:00 , DOI: 10.1021/acs.orglett.6b02072
Donghui Ma 1 , Zhuliang Zhong 1 , Zaimin Liu 1 , Mingjie Zhang 1 , Shiyan Xu 1 , Dengyu Xu 1 , Dengpeng Song 1 , Xingang Xie 1 , Xuegong She 1, 2

从Wade的fawcettimine烯酮仅8步(距离市售(R)-(+)-pulegone步12步)完成了(- )-番茄红素的无保护基路线的全合成。该生物碱的核心骨架是通过磷酸促进的和高度立体控制的炔烃氮杂-普林斯环化反应构建的,同步建立桥接的B环和C13季碳立体中心。该合成方法还具有制备其他番茄红素型生物碱。
06. Visible-Light-Promoted Dual C–C Bond Formations of Alkynoates via a Domino Radical Addition/Cyclization Reaction: A Synthesis of Coumarins
Organic Letters ( IF 6.072 ) Pub Date : 2016-07-22 00:00:00 , DOI: 10.1021/acs.orglett.6b01857
Shangbiao Feng 1 , Xingang Xie 1 , Weiwei Zhang 1 , Lin Liu 1 , Zhuliang Zhong 1 , Dengyu Xu 1 , Xuegong She 1, 2

实现了可见光促进的炔属酸酯的温和条件下的直接双官能化。光氧化还原介导的氧化合成香豆素核心结构以产生α-氧代自由基,该α-氧代自由基在室温下以中等至良好的产率。
07. Asymmetric Total Synthesis of (−)-Lycospidine A
Organic Letters ( IF 6.072 ) Pub Date : 2016-08-26 00:00:00 , DOI: 10.1021/acs.orglett.6b02322
Shiyan Xu 1 , Jing Zhang 1 , Donghui Ma 1 , Dengyu Xu 1 , Xingang Xie 1 , Xuegong She 1, 2

石松生物碱( - ) - lycospidine A,含有前所未有的五元环,这种无保护基的短合成关键是酰胺化/氮杂-Prins多米诺环化反应来快速构建三环骨架和两个连续的立体中心(其中一个是桥接的四级立体中心)。分子内醇醛缩合成功地用于建立独特的五元环,并通过生物合成途径激发的后期氧化反应来合成(-)-糖苷A的二酚环。
08. Palladium-Promoted Neutral 1,4-Brook Rearrangement/Intramolecular Allylic Cyclization Cascade Reaction: A Strategy for the Construction of Vinyl Cyclobutanols
Organic Letters ( IF 6.072 ) Pub Date : 2017-06-09 00:00:00 , DOI: 10.1021/acs.orglett.7b01381
Hao Zhang 1 , Shiqiang Ma 1 , Ziyun Yuan 1 , Peng Chen 1 , Xingang Xie 1 , Xiaolei Wang 1 , Xuegong She 1, 2

该级联反应通过钯活化乙烯基环氧化物,然后进行1,4-Brook重排和与所得碳阴离子的钯配合物进行分子内环化,从而构建乙烯基环丁醇环。通过该级联反应,以高收率和高立体选择性获得了几种高度取代的环丁醇底物。
09. (3 + 2)-Annulation of p-Quinamine and Aryne: A Strategy To Construct the Multisubstituted Hydrocarbazoles
Organic Letters ( IF 6.072 ) Pub Date : 2017-06-28 00:00:00 , DOI: 10.1021/acs.orglett.7b01578
Dengyu Xu 1 , Yulong Zhao 1 , Dengpeng Song 1 , Zhuliang Zhong 1 , Shangbiao Feng 1 , Xingang Xie 1 , Xiaolei Wang 1 , Xuegong She 1, 2

苯二胺和芳烃的(3 + 2)-环化反应,合成多取代的咔唑的策略。可以在温和条件下以令人满意的产率合成具有季碳中心的咔唑的新类似物。
10. Total synthesis of conosilane A via a site-selective C–H functionalization strategy
Chemical Communications ( IF 6.065 ) Pub Date : 2018-01-02 00:00:00 , DOI: 10.1039/c7cc09367e
Ziyun Yuan 1, 2, 3 , Xiaojun Hu 1, 2, 3 , Hao Zhang 1, 2, 3 , Lin Liu 1, 2, 3 , Peng Chen 1, 2, 3 , Min He 1, 2, 3 , Xingang Xie 1, 2, 3 , Xiaolei Wang 1, 2, 3 , Xuegong She 1, 2, 3, 4, 5

通过立体选择性的分子内自由基环化和对位选择性C–H官能化作为关键步骤,实现了高浓度氧化的非异戊二烯基倍半萜烯-甲硅烷C的第一个全合成。
11. Construction of the Tetracyclic Core of Calyciphylline B-Type Daphniphyllum Alkaloids.
Organic Letters ( IF 6.072 ) Pub Date : 2019-10-15 , DOI: 10.1021/acs.orglett.9b03322
Chenglong Du 1 , Jing Fang 1 , Jinyan Chen 1 , Zaimin Liu 1 , Huilin Li 1 , Xiaolei Wang 1 , Xingang Xie 1 , Xuegong She 1

双环化策略来构建茶碱B型生物碱的常见四环核心。合成的关键反应包括不对称的伊文思烷基化,闭环易位反应,分子间酰胺化,分子内氮杂-迈克尔加成反应和醛醇缩合反应。该策略可以应用于这种类型的天然产物的全部合成。
12. Total Synthesis of (-)-Pepluanol B: Conformational Control of the Eight-Membered-Ring System.
Angewandte Chemie International Edition ( IF 16.823 ) Pub Date : 2020-01-02 , DOI: 10.1002/anie.201915876
Jing Zhang 1 , Meng Liu 1 , Chuanhua Wu 1 , Gaoyuan Zhao 1 , Peiqi Chen 1 , Lin Zhou 1 , Xingang Xie 1 , Ran Fang 1 , Huilin Li 1 , Xuegong She 1

外消旋和对映体富集形式的大戟属二萜类萜烯醇B的第一个全合成涉及来自已知双环二醇的20个步骤。用于构建四环骨架的显着反应包括在空间上具有挑战性的羟醛反应以建立四元中心,闭环易位(RCM)形成八元环,以及非对映选择性环丙烷化以组装嵌入的环丙烷基。
13. Total Synthesis of (-)-Gardmultimine A.
Organic Letters ( IF 6.072 ) Pub Date : 2020-02-25 , DOI: 10.1021/acs.orglett.0c00399
Peiqi Chen 1 , Hesi Yang 1 , Hao Zhang 1 , Wei Chen 1 , Zheng Zhang 1 , Jing Zhang 1 , Huilin Li 1 , Xiaolei Wang 1 , Xingang Xie 1 , Xuegong She 1

Gardneria oxindole生物碱(-)-gardmultimine A的第一个全合成反应是从d-色氨酸以完全立体控制的方式通过19个步骤完成的。该合成的特征是:(1)Ir催化的区域选择性C–H硼酸化/氧化序列引入C12甲氧基;(2)吲哚的立体控制氧化重排以构建螺并吲哚基序;(3)Au(I)-催化跨环的Conia-ene型6-exo-dig环化反应,以建立具有独特立体选择性的氮杂双环[2.2.2]辛烷骨架和环外E-烯烃。
14. Collective Total Syntheses of Five Lycopodium Alkaloids
Angewandte Chemie International Edition ( IF 16.823 ) Pub Date : 2022-05-20 , DOI: 10.1002/anie.202205439
Feifei He 1 , Shangbiao Feng 1 , Yulong Zhao 1 , Hongliang Shi 1 , Xiaoguang Duan 1 , Huilin Li 1 , Xingang Xie 1 , Xuegong She 1

五种石松属生物碱的集群式全合成以对映选择性和无保护基团的方式完成。合成包括金催化的烯酰胺-炔烃环异构化和在三环骨架的不同反应位点建立骨架多样化方法,以实现石松属生物碱的不同全合成。
15. Construction of the Skeleton of Lucidumone
Organic Letters ( IF 6.072 ) Pub Date : 2022-07-27 , DOI: 10.1021/acs.orglett.2c02023
Shiqiang Ma 1 , Zhen Li 1 , Pengfei Yu 1 , Hongliang Shi 1 , Hesi Yang 1 , Jiuzhou Yi 1 , Zheng Zhang 1 , Xiaoguang Duan 1 , Xingang Xie 1 , Xuegong She 1

通过氧化去芳构化/分子内 Diels-Alder 反应、Cu 介导的远程 C-H 羟基化、烯丙基氧化、酸促进的动态动力学拆分环化和苄基氧化构建了 lucidumone 的骨架。
16. De Novo Diastereoselective Synthesis of 1-Hydroxyl Allogibberic Methyl Ester en Route to Diverse Bioactive Molecules
Organic Letters ( IF 6.072 ) Pub Date : 2022-08-26 , DOI: 10.1021/acs.orglett.2c02422
Jinyan Chen 1 , Yunxia Yang 1 , Chuanhua Wu 1 , Liang Huo 1 , Xingang Xie 1 , Huilin Li 1 , Xuegong She 1

第一次从头合成 1-羟基同源甲基酯,在生成pharbinilic acid 和其他生物活性分子的过程中,以非对映选择性方式完成。该合成的关键反应包括 Pd 催化的 Suzuki-Miyaura 交叉偶联反应、Lewis 酸催化的还原 Prins 环化反应和 SmI 2介导的跨环频哪醇偶联反应。该合成为获取各种相关生物活性分子提供了新途径。
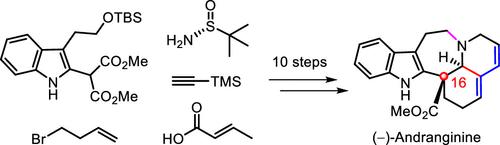
17. Ten-Step Total Synthesis of (−)-Andranginine
Organic Letters ( IF 5.2 ) Pub Date : 2022-09-13 , DOI: 10.1021/acs.orglett.2c02927
Yulong Zhao 1 , Jiaxin Li 1 , Ruize Ma 1 , Feifei He 1 , Hongliang Shi 1 , Xiaoguang Duan 1 , Huilin Li 1 , Xingang Xie 1 , Xuegong She 1
 |
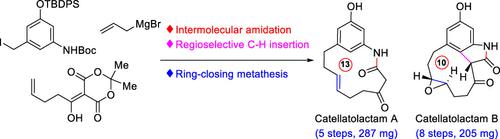 |
 |
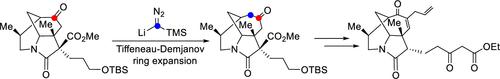 |
21. Rearrangement of the Tetra- and Tricyclic Skeletons of Pepluanol B to Access the Core Structures of Tigliane- and Myrsinane-Type Euphorbia Diterpenes
Organic Letters ( IF 5.2 ) Pub Date : 2023-10-31 , DOI: 10.1021/acs.orglett.3c03109
Chuanhua Wu 1 , Jing Zhang 1 , Meng Liu 1 , Xingang Xie 1 , Huilin Li 1 , Xuegong She 1
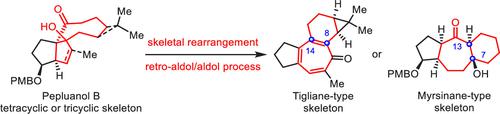 |