热烈祝贺课题组博士生江岭峰题为“Enantioselective copper-catalyzed azidation/click cascade reaction for access to chiral 1,2,3-triazoles”的论文被Nature Communications接收
中文标题:通过铜催化叠氮化/Click串联反应对映选择性合成手性1,2,3-三氮唑
英文标题:Enantioselective copper-catalyzed azidation/click cascade reaction for access to chiral 1,2,3-triazoles
刊物名称及期号、页码: Nat. Commun., 2024, 15, 4919.
作者姓名(中文):江岭峰,吴少华,蒋宇轩,马鸿祥,贺佳俊,毕扬波,孔德意,成轩,邓清海*
作者姓名(英文):Ling-Feng Jiang, Shao-Hua Wu, Yu-Xuan Jiang, Hong-Xiang Ma, Jia-Jun He, Yang-Bo Bi, De-Yi Kong, Yi-Fei Cheng, Xuan Cheng & Qing-Hai Deng*
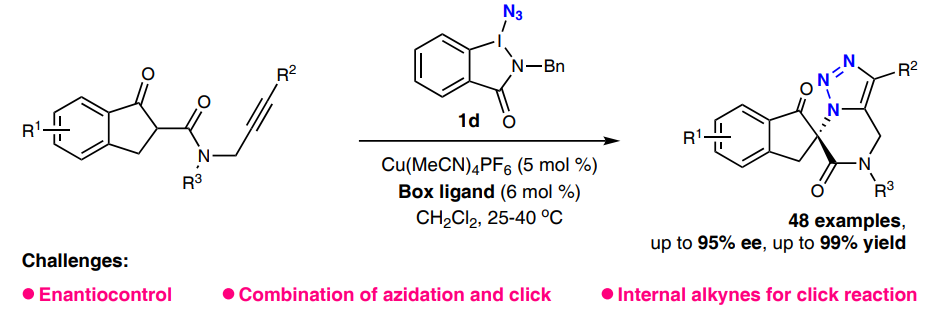
摘要(英文):Chiral 1,2,3-triazoles are highly attractive motifs in various fields. However, achieving catalytic asymmetric click reactions of azides and alkynes for chiral triazole synthesis remains a significant challenge, mainly due to the limited catalytic systems and substrate scope. Herein, we report an enantioselective azidation/click cascade reaction of N-propargyl-β-ketoamides with a readily available and potent azido transfer reagent via copper catalysis, which affords a variety of chiral 1,2,3-triazoles with up to 99% yield and 95% ee under mild conditions. Notably, chiral 1,5-disubstituted triazoles that have not been accessed by previous asymmetric click reactions are also prepared with good functional group tolerance.
工作简介:
邓清海教授课题组一直致力于新型高碘叠氮试剂的合成及不对称叠氮化反应研究,在前期工作的基础上,以酰胺型高碘叠氮试剂为叠氮源,发展了一类铜催化的N-炔丙基-β-酮酰胺不对称叠氮化/点击串联反应,成功实现了1,5-二取代和1,4,5-三取代手性1,2,3-三氮唑化合物的合成。该反应条件温和,官能团容忍度高,对映选择性优异,通过控制实验并结合DFT计算,阐明了该反应可能的机理过程。该合成策略为手性三氮唑化合物的合成提供了新思路。
全文链接:doi.org/10.1038/s41467-024-49313-x