热烈祝贺课题组硕士研究生陈宇轩、霍涛和殷权题为“Azidobenziodazolones as Azido Sources for the Enantioselective Copper-Catalyzed Azidation of N-Unprotected 3-Trifluoromethylated Oxindoles”的论文被Organic Letters接收
中文标题:以高碘叠氮试剂作为叠氮源实现铜催化三氟甲基氧化吲哚的不对称叠氮化
英文标题:Azidobenziodazolones as Azido Sources for the Enantioselective Copper-Catalyzed Azidation of N-Unprotected 3-Trifluoromethylated Oxindoles
刊物名称及期号、页码:Org. Lett. 2023, 25, 15, 2739–2744.
作者姓名(中文):陈宇轩†,霍涛†,殷权†,江岭峰,成轩,蒋宇轩,马鸿祥,孙梅枝,邓清海*
作者姓名(英文):Yu-Xuan Chen,† Tao Huo,† Quan Yin,† Ling-Feng Jiang, Xuan Cheng, Hong-Xiang Ma, Yu-Xuan Jiang, Mei-Zhi Sun, and Qing-Hai Deng*
摘要(英文):Both azido (N3) and trifluoromethyl (CF3) groups are key moieties of numerous valuable molecules that are extensively applied in drug discovery, chemical biology, and synthetic chemistry. However, the asymmetric construction of chiral quaternary stereocenters bearing both N3 and CF3 groups is still unexplored. Herein, we report a kind of bench-stable and easily adjustable benziodazolone-based azidating reagents. These reagents were used to achieve an enantioselective copper-catalyzed azidation of N-unprotected 3-trifluoromethylated oxindoles to provide diverse enantioenriched 3-N3-3-CF3 oxindoles.
工作简介:
上海师范大学邓清海课题组致力于C-N键构建的相关反应方法和机理研究。近日,邓清海教授报道了一类基于高碘叠氮试剂的铜催化三氟甲基氧化吲哚的不对称叠氮化反应。本工作以邻碘苯甲酸为原料,建立了一个含苄基、烷基、环烷基等各种取代基的新型环状酰胺型高碘叠氮试剂库。利用这些叠氮试剂首次实现了铜催化N上无保护基修饰的3-CF3氧化吲哚的对映选择性叠氮化反应,以优秀的产率和较好的对映选择性获得叠氮化产物。该反应条件温和、操作简便且底物适应性良好。初步证明了该叠氮试剂库的实用性,为不对称叠氮化反应的研究提供了新的可能性。
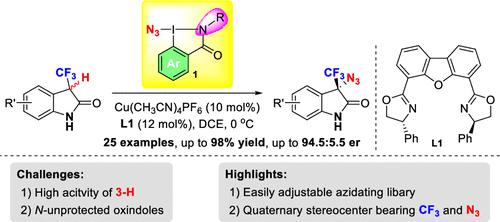
创新点:以邻碘苯甲酸为原料合成了一系列新型环状酰胺型高碘叠氮试剂,基于此类试剂开发了一种铜催化的氮上无保护的三氟甲基氧化吲哚的不对称叠氮化反应。
全文链接:https://doi.org/10.1021/acs.orglett.3c01005