Julian Wilke, Tatsuro Kawamura, Hao Xu, Alexandra Brause, Alexandra Friese, Malte Metz, Dirk Schepmann, Bernhard Wünsch, Antonia Artacho-Cordón, Francisco R.Nieto, Nobumoto Watanabe, Hiroyuki Osada, Slava Ziegler, Herbert Waldmann*
Cell Chemical Biology.2021, Doi.org/10.1016/j.chembiol.2021.01.009 https://www.sciencedirect.com/science/article/pii/S245194562100009X?via%3Dihub 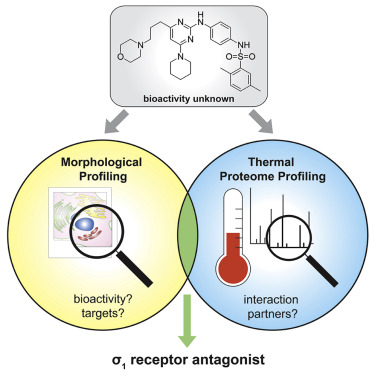
Si-Jia Li§, Jian Huang§, Jin-Yu He, Rui-Jin Zhang, Hao-Dong Qian, Xue-Lin Dai, Han-Han Kong and Hao Xu*
Highly enantioselective copper-catalyzed propargylic amination to access N-tethered 1,6-enynes.
RSC Adv., 2020, 10, 38478. https://pubs.rsc.org/en/content/articlelanding/ 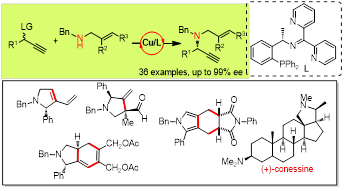 A highly enantioselective copper-catalyzed propargylic amination starting from benzylic allylic amines has been developed with a new chiral N,N,P ligand, providing a series of N-tethered 1,6-enynes in good to excellent yields with excellent enantioselectivities.
A highly enantioselective copper-catalyzed propargylic amination starting from benzylic allylic amines has been developed with a new chiral N,N,P ligand, providing a series of N-tethered 1,6-enynes in good to excellent yields with excellent enantioselectivities.
Hao Xu, Luca Laraia, Laura Schneider, Kathrin Louven, Carsten Strohmann, Andrey P. Antonchick* and Herbert Waldmann*;
Angew. Chem. Int. Ed. 2017, 56, 11232. https://onlinelibrary.wiley.com/doi/full/10.1002/anie.201706005
(Highlighted by Synfacts. 2017, 13, 1047. ) https://www.thieme-connect.de/products/ejournals/abstract/10.1055/s-0036-1591260 、 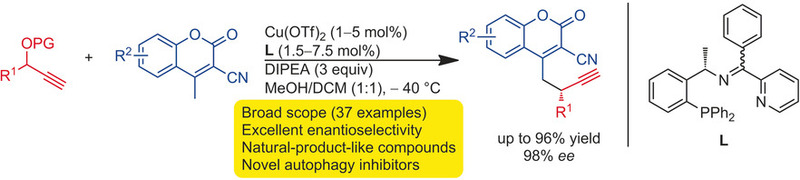
Hao Xu, Christopher Golz, Carsten Strohmann, Andrey P. Antonchick* and Herbert Waldmann*;
Angew. Chem. Int. Ed. 2016, 55, 7761. https://onlinelibrary.wiley.com/doi/full/10.1002/anie.201602084 
Hao Xu§, Jiang-Lin Hu§, Li-Jia Wang, Sai-Hu Liao and Yong Tang*; J. Am. Chem. Soc. 2015, 137, 8006. https://pubs.acs.org/doi/10.1021/jacs.5b04429
Asymmetric Annulation of Donor−Acceptor Cyclopropanes with Dienes. (§: contributed equally)
(Highlighted by Synfacts. 2015, 11, 956.) https://www.thieme-connect.de/products/ejournals/abstract/10.1055/s-0035-1560117
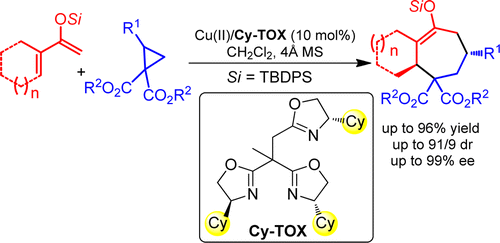
Jiang-Lin Hu, Li-Jia Wang, Hao Xu, Zuo-Wei Xie* and Yong Tang*; Org. Lett. 2015, 17, 2680. https://pubs.acs.org/doi/10.1021/acs.orglett.5b01077
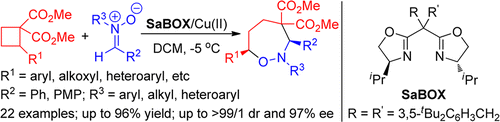
Hao Xu§, Jian-Ping Qu§, Sai-Hu Liao, Hu Xiong and Yong Tang*; Angew. Chem. Int. Ed. 2013, 52, 4004.
(Selected as a Hot paper) https://onlinelibrary.wiley.com/doi/full/10.1002/anie.201300032
(Highlighted by Synfacts. 2013, 9, 648.)https://www.thieme-connect.de/products/ejournals/abstract/10.1055/s-0033-1338829
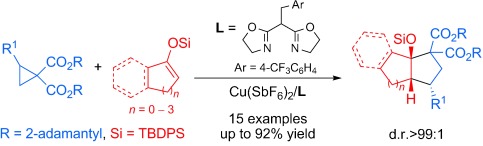
Highly Enantioselective [3+2] Annulation of Cyclic Enol Silyl Ethers with Donor–Acceptor Cyclopropanes: Accessing 3a-Hydroxy [n.3.0]Carbobicycles. ( §: contributed equally)
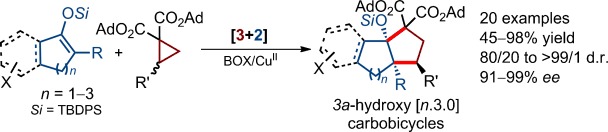
Hu Xiong§, Hao Xu§, Sai-Hu Liao, Zuo-Wei Xie* and Yong Tang*;
J. Am. Chem. Soc. 2013, 135, 7851.https://pubs.acs.org/doi/10.1021/ja4042127
( §: contributed equally)
(Highlighted by Synform, 2013, 7, 122.)
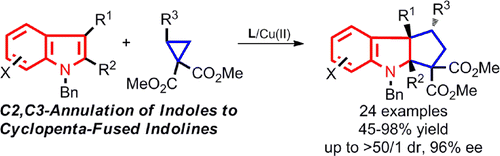
Jiao-Long Zhou, Li-Jia Wang, Hao Xu, Xiu-Li Sun and Yong Tang*; ACS Catalysis. 2013, 3, 685. https://pubs.acs.org/doi/10.1021/cs400019u
Jian-Ping Qu, Yong Liang, Hao Xu, Xiu-Li Sun, Zhi-Xiang Yu* and Yong Tang*
Highly Diastereoselective Construction of Fused Carbocycles from Cyclopropane-1,1-dicarboxylates and Cyclic Enol Silyl Ethers: Scope, Mechanism, and Origin of Diastereoselectivity
Chem. Eur. J. 2012, 18, 2196.https://chemistry-europe.onlinelibrary.wiley.com/doi/full/10.1002/chem.201103495