36. Xin-Yu Zhang+, Deng Zhu+, Ren-Fei Cao, Yu-Xuan Huo, Tong-Mei Ding, Zhi-Min Chen*
Enantioselective Synthesis of Inherently Chiral Sulfur-Containing Calix[4]arenes via Chiral Sulfide Catalyzed Desymmetrizing Aromatic Sulfenylation
Nat. Commun. 2024, 15, 9929.

35. Yu-Hang Zhao (本科生), Deng Zhu, Zhi-Min Chen*
ChemCatChem 2024, e202401312, DOI: 10.1002/cctc.202401312, accepted.

34. Qin Yang, Hui-Yun Luo, Deng Zhu, Xin-Yu Zhang, Hua Ke*, Zhi-Min Chen*
Chiral Lewis Base/Achiral Acid Co-Catalyzed Atroposelective Sulfenylation of Pyrrole Derivatives: Construction of C-N Axially Chiral Sulfides
Chin. J. Chem. 2024, 42, 2005-2009.

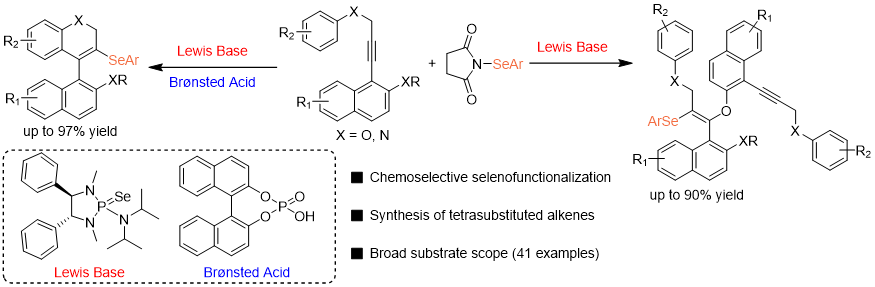
33. Ling-Ling Chen,† Ren-Fei Cao,† Hua Ke*, Zhi-Min Chen*
Lewis Base Catalyzed Selenofunctionalization of Alkynes with Acid-controlled Divergent Chemoselectivity
Chin. J. Chem. 2024, 42, 1623-1629. (invited submission for 2024 Emerging Investigator Issue)
32. Deng Zhu, Tong Mu, Ze-Long Li, Hui-Yun Luo, Ren-Fei Cao, Xiao-Song Xue*, Zhi-Min Chen*
Enantioselective Synthesis of Planar-Chiral Sulfur-Containing Cyclophanes by Chiral Sulfide Catalyzed Electrophilic Sulfenylation of Arenes.
Angew. Chem. Int. Ed. 2024, 63, e202318625.
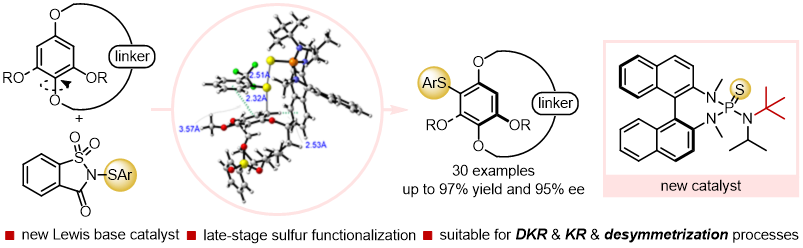
31. Deng Zhu+, Lu Yu+, Hui-Yun Luo, Xiao-Song Xue*, Zhi-Min Chen*
Atroposelective Electrophilic Sulfenylation of N-Aryl Aminoquinone Derivatives Catalyzed by Chiral SPINOL-Derived Sulfide
Angew. Chem. Int. Ed. 2022, 61, e202211782 (Hot Paper).
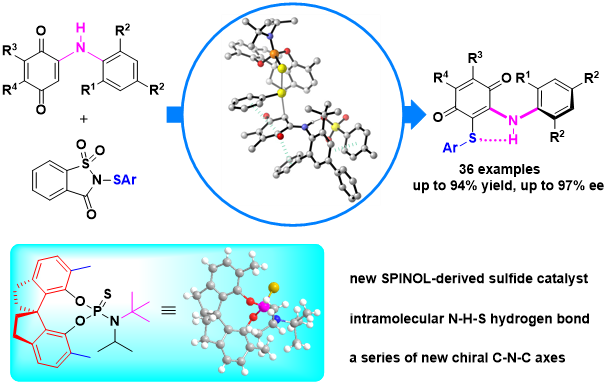
30. Hui-Yun Luo, Zi-Hao Li, Deng Zhu, Qin Yang, Ren-Fei Cao, Tong-Mei Ding, Zhi-Min Chen*
Chiral Selenide/Achiral Sulfonic Acid Co-catalyzed Atroposelective Sulfenylation of Biaryl Phenols via a Desymmetrization/Kinetic Resolution Sequence
J. Am. Chem. Soc. 2022, 144, 2943-2952.

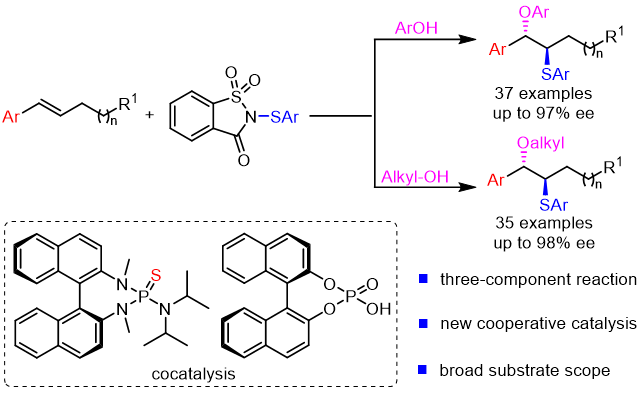
29. Xiao-Dong Liu+, Yicong Luo+, Xiaohong, Huo, Hui-Yun Luo, Ren-Fei Cao, Zhi-Min Chen*
Chiral Sulfide/Phosphoric Acid Cocatalyzed Enantioselective Intermolecular Oxysulfenylation of Alkenes with Phenol and Alcohol O-nucleophiles
CCS Chem., 2022, 4, 3342-3354.
28. Yu-Yang Xie, Zhi-Min Chen,* Hui-Yun Luo, Hui Shao, Yong-Qiang Tu,* Xiaoguang Bao,* Ren-Fei Cao, Shu-Yu Zhang, Jin-Miao Tian
Lewis Base/Brønsted Acid Cocatalyzed Enantioselective Sulfenylation/Semipinacol Rearrangement of Di- and Trisubstituted Allylic Alcohols
Angew. Chem. Int. Ed. 2019, 58, 12491-12496. Featured by Benjamin List, Joyce A. A. Grimm in SYNFACTS 2019, 15, 1175.
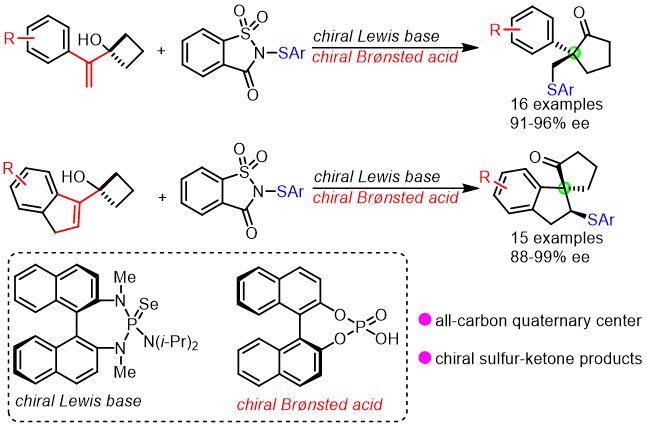
27. Xiao-Dong Liu, Ai-Hui Ye, Zhi-Min Chen*
Catalytic Enantioselective Intermolecular Three-Component Sulfenylative Difunctionalizations of 1,3-Dienes
ACS Catal. 2023, 13, 2715-2722.
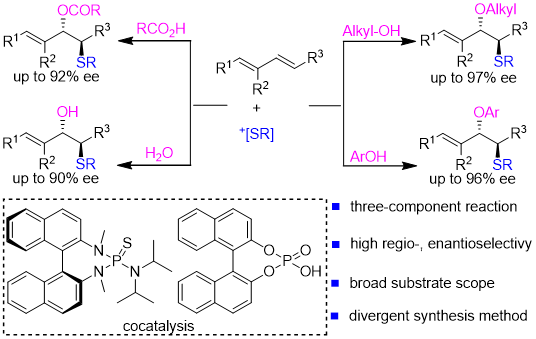
26. Ren-Fei Cao, Zhi-Min Chen*
Catalytic asymmetric synthesis of sulfur-containing atropisomers by C−S bond formations
Sci. China Chem. 2023, Accepted. (invited submission for 2023 Emerging Investigator Issue)
25. Xin-Yu Zhang+, Deng Zhu+, Yu-Xuan Huo, Ling-Ling Chen, Zhi-Min Chen*
Atroposelective Sulfenylation of Biaryl Anilines Catalyzed by Chiral SPINOL-Derived Selenide
Org. Lett. 2023, 25, 3445-3450.
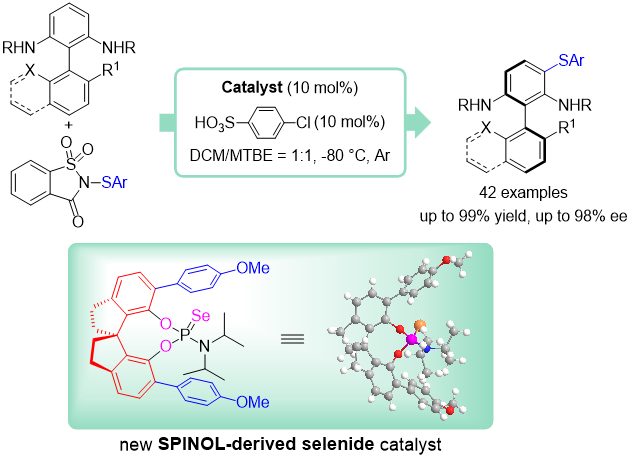
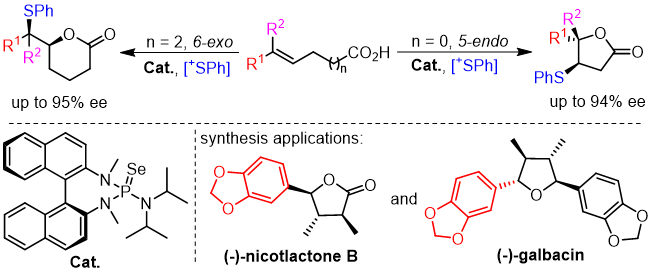
24. Hui-Yun Luo, Yu-Yang Xie, Xu-Feng Song, Jia-Wei Dong, Deng Zhu, Zhi-Min Chen*
Lewis base-catalyzed asymmetric sulfenylation of alkenes: construction of sulfenylated lactones and application to the formal syntheses of (-)-nicotlactone B and (-)-galbacin
Chem. Commun., 2019, 55, 9367-9370.
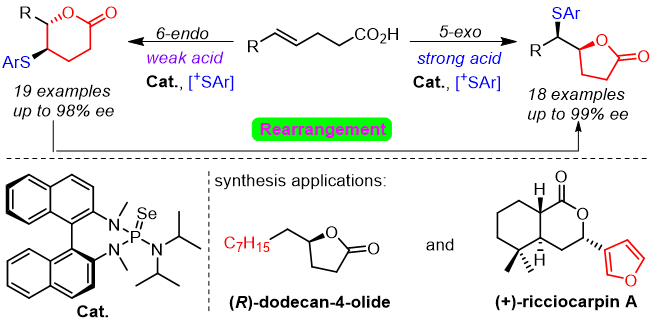
23. Hui-Yun Luo, Jia-Wei Dong, Yu-Yang Xie, Xu-Feng Song, Deng Zhu, Tongmei Ding, Yuanyuan Liu,* Zhi-Min Chen*
Lewis Base/Brønsted Acid Co-catalyzed Asymmetric Thiolation of Alkenes with Acid-Controlled Divergent Regioselectivity
Chem. Eur. J. 2019, 25, 15411-15418.
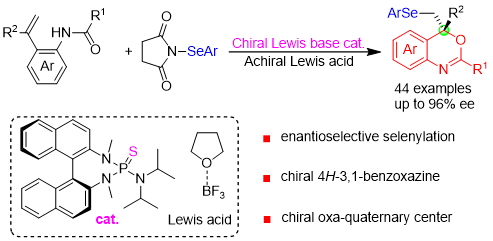
22. Ren-Fei Cao+, Lu Yu+, Yu-Xuan Huo, Yao Li, Xiao-Song Xue, Zhi-Min Chen*
Chiral Lewis Base Catalyzed Enantioselective Selenocyclization of 1,1-Disubstituted Alkenes: Asymmetric Synthesis of Selenium-Containing 4H-3,1-Benzoxazines
Org. Lett. 2022, 24, 4093-4098.
21. Deng Zhu, Zhi-Min Chen*
Application of Chiral Lewis Base/Brønsted Acid Synergistic Catalysis Strategy in Enantioselective Synthesis of Organic Sulfides
Chin. J. Org. Chem. 2022, 42, 3015-3032. (invited submission)
20. Deng Zhu, Ai-Hui Ye, Zhi-Min Chen*
N-Selenocyanato-dibenzenesulfonimide: a new electrophilic selenocyanation reagent (Feature Article, invited submission)
Synthesis 2021, 53, 3744-3750.

19. Ai-Hui Ye, Ye Zhang, Yu-Yang Xie, Hui-Yun Luo, Jia-Wei Dong, Xiao-Dong Liu, Xu-Feng Song, Tongmei Ding, Zhi-Min Chen*
TMSCl-Catalyzed Electrophilic Thiocyano Oxyfunctionalization of Alkenes Using N-Thiocyano-dibenzenesulfonimide
Org. Lett., 2019, 21, 5106-5110.

18. Xu-Feng Song,† Ai-Hui Ye,† Yu-Yang Xie, Jia-Wei Dong, Chao Chen, Ye Zhang,* Zhi-Min Chen* (†equal contribution)
Lewis Acid Mediated Thiocyano Semipinacol Rearrangement of Allylic Alcohols for the Construction of α-Quaternary Center β-Thiocyano Carbonyls
Org. Lett., 2019, 21, 9550-9554.

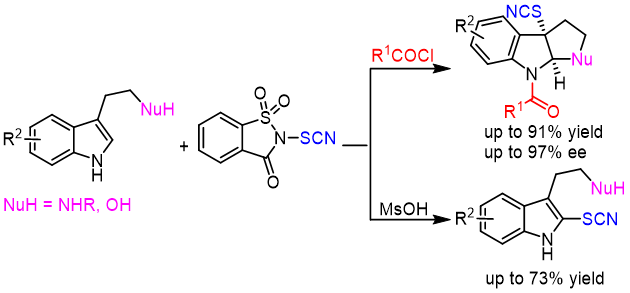
17. Ai‐Hui Ye, Xu-Feng Song, Zhi‐Min Chen*
Electrophilic Thiocyanation of Tryptamine Derivatives: Divergent Synthesis of SCN-Containing Indole Compounds
Chem. Asian J. 2022, 17, e202200802 (10.1002/asia.202200802).
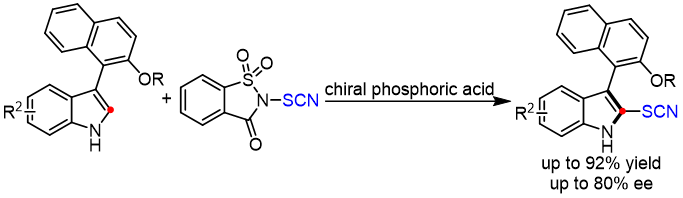
16. Ai‐Hui Ye, Zi-Hao Li, Tong-Mei Ding, Hua Ke*, Zhi‐Min Chen*
Phosphoric Acid Catalyzed Electrophilic Thiocyanation of Indoles: Access to SCN-Containing Aryl-Indole Compounds
Chem. Asian J. 2022, 17, e202200256, (DOI: 10.1002/asia.202200256).
15. Kai Ji, Ka Lu, Jie Huang, Zi-Hao Li, Tong-Mei Ding, Zhi-Min Chen*
Brønsted Acid-Catalyzed Solvent-Controlled Regioselective Hydrothiolation and Diastereoselective Cascade Cyclization of Dienes
Chem. Commun., 2021, 57, 12639-12642.
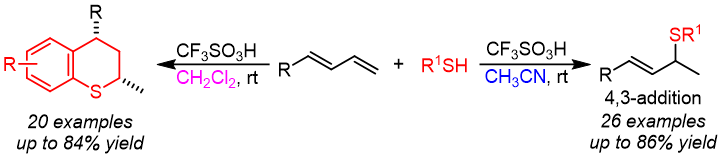
14. Kai Ji, Ka Lu, Jie Huang, Zi-Hao Li, Hua Ke*, Zhi-Min Chen*
Brønsted Acid-Catalyzed Regioselective Hydrothiolation of Dienes: Solvent-Controlled Divergent Synthesis of Sulfides
Org. Lett. 2021, 23, 8028-8032.

13. Deng Zhu, Hui-Yun Luo, Zhi-Min Chen*
Selenium-Catalyzed Trifluoromethylsulfinylation/Rearrangement of Allylic and Propargylic Alcohols: Access to Allylic and Allenic Triflones
Org. Lett. 2021, 23, 1044-1048.
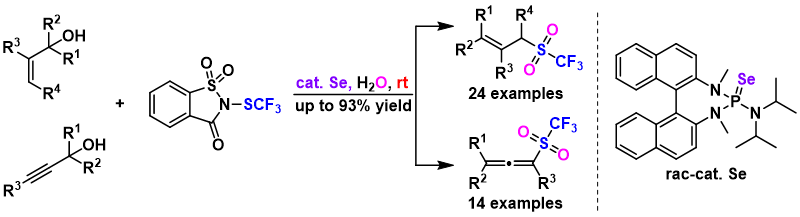
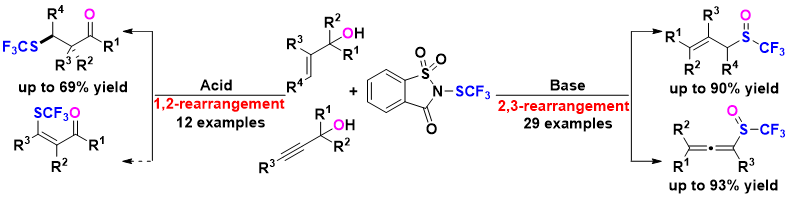
12. Deng Zhu, Tong-Mei Ding, Hui-Yun Luo, Hua Ke, Zhi-Min Chen*
Divergent Synthesis of Trifluoromethyl Sulfoxides and β-SCF3 Carbonyl Compounds by Tandem Trifluoromethylthiolation/Rearrangement of Allylic and Propargylic Alcohols
Org. Lett. 2020, 22, 7699-7703.
11. Xu-Feng Song, Tong-Mei Ding, Deng Zhu, Jie Huang, Zhi-Min Chen*
Lewis-Acid-Mediated Intramolecular Trifluoromethylthiolation of Alkenes with Phenols: Access to SCF3‑Containing Chromane and Dihydrobenzofuran Compounds
Org. Lett. 2020, 22, 7052-7056.

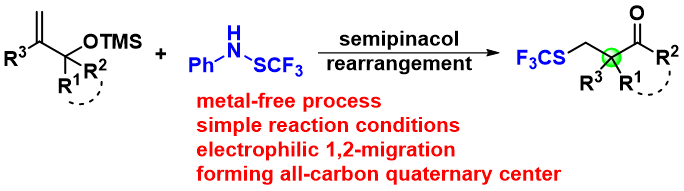
10. Chao-Chao Xi, Zhi-Min Chen*, Shu-Yu Zhang, Yong-Qiang Tu*.
Electrophilic Trifluoromethylthiolation/Semipinacol Rearrangement: Preparation of β-SCF3 Carbonyl Compounds with α-Quaternary Carbon Center.
Org. Lett., 2018, 20, 4227–4230.
9. Kai Ji†, Jie Huang†, Xin-Yu Zhang, Zhi-Min Chen*
Enantioselective 1,1-Diarylation of Allyl Sulfones Catalyzed by Palladium with a Chiral Phosphoric Acid
Chem Synth 2022;2:17. http://dx.doi.org/10.20517/cs.2022.27.
8. Jie Huang, Zhi-Min Chen*
The alkynylative difunctionalization of alkenes
Chem. Eur. J. 2022, 28, e202201519.
7. Jie Huang, Ling-Ling Chen, Tong-Mei Ding*, Zhi-Min Chen*
Iron-Catalyzed Hydroarylation of 1,3-Dienes with Indoles
Org. Chem. Front., 2023, 10, 461-466.
6. Jie Huang, Ling-Ling Chen, Zhi-Min Chen*
Palladium-Catalyzed Three-Component Cross-Coupling of Conjugated Dienes with Indoles Using Ethynylbenziodazolones as Electrophilic Alkynylating Reagents
Org. Lett. 2022, 24, 5777-5781.
5. Chao Chen, Jun-Chen Kang, Chen Mao, Jia-Wei Dong, Yu-Yang Xie, Tong-Mei Ding, Yong-Qiang Tu, Zhi-Min Chen*, Shu-Yu Zhang*
Electrochemical Halogenation/Semi-pinacol Rearrangement of Allylic Alcohols Using Inorganic Halide Salt: An Eco-friendly Route to Synthesis of β-halocarbonyls
Green Chem., 2019, 21, 4014-4019 (Inside front cover).
4. Jun-Chen Kang, Yong-Qiang Tu, Jia-Wei Dong, Chao Chen, Jia Zhou, Tong-Mei Ding, Jian-Tao Zai, Zhi-Min Chen*, Shu-Yu Zhang*.
Electrochemical semipinacol rearrangements of allylic alcohols: construction of all-carbon quaternary stereocenters.
Org. Lett., 2019, 21, 2536–2540 (Most downloaded).
3. Yu-Yang Xie, Yun-Peng Wang, Xiao-Jing Zhao, Ai-Fang Wang, Zhi-Min Chen*, Yong-Qiang Tu*.
Oxyallyl Cation Promoted Dearomative Semipinacol Rearrangement: Facile Stereodivergent Synthesis of Spiro-indolines with Contiguous Quaternary Centers
Chem. Commun., 2021, 57, 6632-6635.
2. Chao-Chao Xi, Xiao-Jing Zhao, Jin-Miao Tian*, Zhi-Min Chen*, Kun Zhang, Fu-Min Zhang, Yong-Qiang Tu*, and Jia-Wei Dong
Atroposelective Synthesis of Axially Chiral 3-Arylindoles by Copper-Catalyzed Asymmetric Cross-Coupling of Indoles with Quinones and Naphthoquinones
Org. Lett. 2020, 22, 4995-5000.
1. Jia‐Wei Dong, Tongmei Ding, Shu‐Yu Zhang*, Zhi‐Min Chen*, Yong‐Qiang Tu*.
A Facile Approach to Oximes and Ethers by a Tandem NO+‐Initiated Semipinacol Rearrangement and H‐Elimination.
Angew. Chem. Int. Ed. 2018, 57, 13192-13196.
Prior to SJTU:
8. Zhi-Min Chen†, Jianbo Liu†, Jing-Yao Guo, Maximilian Loch, Ryan Deluca, Matthew S. Sigman* (†equal contribution)
Palladium-catalyzed enantioselective alkenylation of alkenylbenzene derivatives
Chem. Sci., 2019, 10, 7246-7250.
7. Zhi-Min Chen, Christine S. Nervig, Ryan J. DeLuca, and Matthew S. Sigman*. Palladium-catalyzed enantioselective redox-relay Heck alkynylation of alkenols to access propargylic stereocenters
Angew. Chem. Int. Ed. 2017, 56, 6651-6654.
6. Zhi-Min Chen, Margaret J. Hilton, and Matthew S. Sigman*.
Palladium-catalyzed enantioselective redox-relay Heck arylation of 1,1-disubstituted homoallylic alcohols
J. Am. Chem. Soc. 2016, 138, 11461-11464.
5. Zhi-Min Chen,† Xiao-Ming Zhang†, and Yong-Qiang Tu* (†equal contribution). Radical aryl migration reactions and synthetic applications
Chem. Soc. Rev. 2015, 44, 5220-5245.
4. Zhi-Min Chen, Zhen Zhang, Yong-Qiang Tu,* Ming-Hui Xu, Fu-Min Zhang, Chen-Chen Li, and Shao-Hua Wang*.
A Mn(III)/TEMPO-co-mediated tandem azidation/1,2-carbon migration reaction of allylic silyl ethers
Chem. Commun. 2014, 50, 10805-10808.
3. Zhi-Min Chen, Wei Bai, Shao-Hua Wang, Bin-Miao Yang, Yong-Qiang Tu,* and Fu-Min Zhang.
Copper-catalyzed tandem trifluoromethylation/semipinacol rearrangement of allylic alcohols
Angew. Chem. Int. Ed. 2013, 52, 9781-9785.
2. Zhi-Min Chen, Bin-Miao Yang, Zhi-Hua Chen, Qing-Wei Zhang, Min Wang, and Yong-Qiang Tu*.
Organocatalytic asymmetric fluorination/semipinacol rearrangement: An efficient approach to chiral β-fluoroketones
Chem. Eur. J. 2012, 18, 12950-12954.
1. Zhi-Min Chen, Qing-Wei Zhang, Zhi-Hua Chen, Hui Li, Yong-Qiang Tu,* Fu-Min Zhang, and Jin-Miao Tian.
Organocatalytic asymmetric halogenation/semipinacol rearrangement: highly efficient synthesis of chiral α-oxa-quaternary β-haloketones
J. Am. Chem. Soc. 2011, 133, 8818-8821.