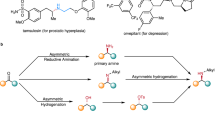
Abstract
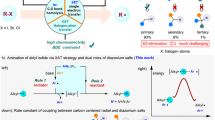
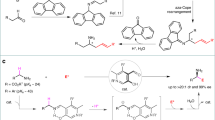
Despite the fact that nucleophilic displacement (SN2) of alkyl halides with nitrogen nucleophiles is one of the first reactions introduced in organic chemistry teaching, its practical utilization is largely limited to unhindered (primary) or activated (α-carbonyl, benzylic) substrates. Here, we demonstrate an alternative amination strategy where alkyl iodides are used as radical precursors instead of electrophiles. Use of α-aminoalkyl radicals enables the efficient conversion of the iodides into the corresponding alkyl radical by halogen-atom transfer, while copper catalysis assembles the sp3 C–N bonds at room temperature. The process provides SN2-like programmability, and application in late-stage functionalization of several densely functionalized pharmaceuticals demonstrates its utility in the preparation of valuable N-alkylated drug analogues.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the paper and its Supplementary Information or from the authors upon reasonable request.
References
Ricci, A. Amino Group Chemistry: From Synthesis to the Life Sciences (Wiley-VCH, (2008).
Vitaku, E., Smith, D. T. & Njardarson, J. T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J. Med. Chem. 57, 10257–10274 (2014).
Brown, D. G. & Boström, J. Analysis of past and present synthetic methodologies on medicinal chemistry: where have all the new reactions gone? J. Med. Chem. 59, 4443–4458 (2016).
Trowbridge, A., Walton, S. M. & Gaunt, M. J. New strategies for the transition-metal catalyzed synthesis of aliphatic amines. Chem. Rev. 120, 2613–2692 (2020).
Kaga, A. & Chiba, S. Engaging radicals in transition metal-catalyzed cross-coupling with alkyl electrophiles: recent advances. ACS Catal. 7, 4697–4706 (2017).
Afanasyev, O. I., Kuchuk, E., Usanov, D. L. & Chusov, D. Reductive amination in the synthesis of pharmaceuticals. Chem. Rev. 119, 11857–11911 (2019).
Kumar, R., Flodén, N. J., Whitehurst, W. G. & Gaunt, M. J. A general carbonyl alkylative amination for tertiary amine synthesis. Nature 581, 415–420 (2020).
Hossain, A., Bhattacharyya, A. & Reiser, O. Copper’s rapid ascent in visible-light photoredox catalysis. Science 364, eaav9713 (2019).
Kochi, J. K. & Subramanian, R. V. Kinetics of electron-transfer oxidation of alkyl radicals by copper(ii) complexes. J. Am. Chem. Soc. 87, 4855–4866 (1965).
Casitas, A. & Ribas, X. The role of organometallic copper(iii) complexes in homogeneous catalysis. Chem. Sci. 4, 2301–2318 (2013).
Zhu, X. & Chiba, S. Copper-catalyzed oxidative carbon–heteroatom bond formation: a recent update. Chem. Soc. Rev. 45, 4504–4523 (2016).
Jacobson, R. R., Tyeklár, Z. & Karlin, K. D. Reaction of organic halides with [CuI(TMPA)CH3CN]PF6. Inorg. Chim. Acta 181, 111–118 (1991).
Cheng, L.-J. & Mankad, N. P. C–C and C–X coupling reactions of unactivated alkyl electrophiles using copper catalysis. Chem. Soc. Rev. 49, 8036–8064 (2020).
Matsumoto, Y. et al. Amino acid Schiff base bearing benzophenone imine as a platform for highly congested unnatural α-amino acid synthesis. J. Am. Chem. Soc. 142, 8498–8505 (2020).
Ishida, S., Takeuchi, K., Taniyama, N., Sunada, Y. & Nishikata, T. Copper-catalyzed amination of congested and functionalized α-bromocarboxamides with either amines or ammonia at room temperature. Angew. Chem. Int. Ed. 56, 11610–11614 (2017).
Bissember, A. C., Lundgren, R. J., Creutz, S. E., Peters, J. C. & Fu, G. C. Transition-metal-catalyzed alkylations of amines with alkyl halides: photoinduced, copper-catalyzed couplings of carbazoles. Angew. Chem. Int. Ed. 52, 5129–5133 (2013).
Kainz, Q. M. et al. Asymmetric copper-catalyzed C–N cross-couplings induced by visible light. Science 351, 681–684 (2016).
Creutz, S. E., Lotito, K. J., Fu, G. C. & Peters, J. C. Photoinduced Ullmann C–N coupling: demonstrating the viability of a radical pathway. Science 338, 647–651 (2012).
Do, H.-Q., Bachman, S., Bissember, A. C., Peters, J. C. & Fu, G. C. Photoinduced, copper-catalyzed alkylation of amides with unactivated secondary alkyl halides at room temperature. J. Am. Chem. Soc. 136, 2162–2167 (2014).
Mao, R., Frey, A., Balon, J. & Hu, X. Decarboxylative C(sp3)–N cross-coupling via synergetic photoredox and copper catalysis. Nat. Catal. 1, 120–126 (2018).
Liang, Y., Zhang, X. & MacMillan, D. W. C. Decarboxylative sp3 C–N coupling via dual copper and photoredox catalysis. Nature 559, 83–88 (2018).
Nguyen, V. T. et al. Visible-light-enabled direct decarboxylative N-alkylation. Angew. Chem. Int. Ed. 59, 7921–7927 (2020).
Constantin, T. et al. Aminoalkyl radicals as halogen-atom transfer agents for activation of alkyl and aryl halides. Science 367, 1021–1026 (2020).
Constantin, T. et al. A case of chain propagation: α-aminoalkyl radicals as initiators for aryl radical chemistry. Chem. Sci. 11, 12822–12828 (2020).
Neff, R. K. et al. Generation of halomethyl radicals by halogen atom abstraction and their addition reactions with alkenes. J. Am. Chem. Soc. 141, 16643–16650 (2019).
Su, Y. L. et al. α-Amino radical-mediated diverse difunctionalization of alkenes: construction of C−C, C−N and C−S bonds. ACS Catal. 10, 13682–13687 (2020).
Tedder, J. M. The importance of polarity, bond strength and steric effects in determining the site of attack and the rate of free radical substitution in aliphatic compounds. Tetrahedron 38, 313–329 (1982).
Kharasch, M. S. & Sosnovsky, G. The reactions of t-butyl perbenzoate and olefins—a stereospecific reaction. J. Am. Chem. Soc. 80, 756–756 (1958).
Wayner, D. D. M., Dannenberg, J. J. & Griller, D. Oxidation potentials of α-aminoalkyl radicals: bond dissociation energies for related radical cations. Chem. Phys. Lett. 131, 189–191 (1986).
Lalevée, J., Allonas, X. & Fouassier, J.-P. N−H and α(C−H) bond dissociation enthalpies of aliphatic amines. J. Am. Chem. Soc. 124, 9613–9621 (2002).
Tran, B. L., Li, B., Driess, M. & Hartwig, J. F. Copper-catalyzed intermolecular amidation and imidation of unactivated alkanes. J. Am. Chem. Soc. 136, 2555–2563 (2014).
Wang, C.-S., Wu, X.-F., Dixneuf, P. H. & Soulé, J.-F. Copper-catalyzed oxidative dehydrogenative C(sp3)−H bond amination of (cyclo)alkanes using NH-heterocycles as amine sources. ChemSusChem 10, 3075–3082 (2017).
Chatgilialoglu, C., Ferreri, C., Landais, Y. & Timokhin, V. I. Thirty years of (TMS)3SiH: a milestone in radical-based synthetic chemistry. Chem. Rev. 118, 6516–6572 (2018).
Zhang, P., Le, C. C. & MacMillan, D. W. C. Silyl radical activation of alkyl halides in metallaphotoredox catalysis: a unique pathway for cross-electrophile coupling. J. Am. Chem. Soc. 138, 8084–8087 (2016).
Stein, S. E. & Brown, R. L. Prediction of carbon–hydrogen bond dissociation energies for polycyclic aromatic hydrocarbons of arbitrary size. J. Am. Chem. Soc. 113, 787–793 (1991).
Zhu, X.-Q. et al. Determination of the C4–H bond dissociation energies of NADH models and their radical cations in acetonitrile. Chem. Eur. J. 9, 871–880 (2003).
Wiese, S. et al. Catalytic C–H amination with unactivated amines through copper(ii) amides. Angew. Chem. Int. Ed. 49, 8850–8855 (2010).
Ahn, J. M., Ratani, T. S., Hannoun, K. I., Fu, G. C. & Peters, J. C. Photoinduced, copper-catalyzed alkylation of amines: a mechanistic study of the cross-coupling of carbazole with alkyl bromides. J. Am. Chem. Soc. 139, 12716–12723 (2017).
Wang, M. et al. [11C]enzastaurin, the first design and radiosynthesis of a new potential PET agent for imaging of protein kinase C. Bioorg. Med. Chem. Lett. 21, 1649–1653 (2011).
Wang, Y. et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 395, 1569–1578 (2020).
Mao, R., Balon, J. & Hu, X. Cross-coupling of alkyl redox-active esters with benzophenone imines: tandem photoredox and copper catalysis. Angew. Chem. Int. Ed. 57, 9501–9504 (2018).
Peacock, D. M., Roos, C. B. & Hartwig, J. F. Palladium-catalyzed cross coupling of secondary and tertiary alkyl bromides with a nitrogen nucleophile. ACS Cent. Sci 2, 647–652 (2016).
Carreira, E. M. & Fessard, T. C. Four-membered ring-containing spirocycles: synthetic strategies and opportunities. Chem. Rev. 114, 8257–8322 (2014).
Lovering, F., Bikker, J. & Humblet, C. Escape from Flatland: increasing saturation as an approach to improving clinical success. J. Med. Chem. 52, 6752–6756 (2009).
Acknowledgements
D.L. thanks EPSRC for a fellowship (EP/P004997/1) and a research grant (EP/T016019/1) and the European Research Council for a research grant (758427). We thank W. Ashworth, P. Gillespie and S. Wells for performing safety studies. We thank M. Johansson (AstraZeneca) for useful discussions.
Author information
Authors and Affiliations
Contributions
F.J. and D.L. designed the project and directed the work. B.G. and A.-L.B. performed all the synthetic and mechanistic experiments. J.J.D performed the scale-up experiments. All authors analysed the results and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Catalysis thanks Michael Doyle and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary methods, discussion, Tables 1–8, Figs. 1–30 and references.
Rights and permissions
About this article
Cite this article
Górski, B., Barthelemy, AL., Douglas, J.J. et al. Copper-catalysed amination of alkyl iodides enabled by halogen-atom transfer. Nat Catal 4, 623–630 (2021). https://doi.org/10.1038/s41929-021-00652-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-021-00652-8