Abstract
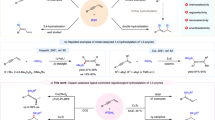
In contrast with abundant methods for the asymmetric functionalization of alkyl radicals to generate stereogenic centres at reaction sites, catalytic enantioselective desymmetrizing functionalization of alkyl radicals for forging multiple stereocentres—including positions that are remote from the reaction sites—with both high enantio- and diastereoselectivity remains largely unexplored. The major challenge for such reactions is the high reactivity of open-shell alkyl radicals. Here, we describe a strategy to address this challenge: the use of Cu(ii) phosphate to immediately associate with the in situ-generated reactive alkyl radical species, creating a compact and confined chiral microenvironment for effective stereocontrol. With this strategy, we have developed a general and efficient catalytic enantioselective desymmetrizing functionalization of alkene-tethered 1,3-diols. It provides various tetrahydrofurans and analogues bearing multiple stereocentres with remarkably high levels of enantio- and diastereocontrol. Density functional theory calculations and mechanistic experiments revealed a reaction mechanism involving an enantiodetermining outer-sphere C–O bond formation step.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Data relating to the materials and methods, optimization studies, experimental procedures, mechanistic studies and DFT calculations, high-performance liquid chromatography spectra, NMR spectra and mass spectrometry are available in the Supplementary Information. Crystallographic data for compounds 3A′, 6 and 8A are available free of charge from the Cambridge Crystallographic Data Centre under reference numbers 1916711 (3A′), 1922870 (6) and 1916714 (8A). All other data are available from the authors upon reasonable request.
References
Sibi, M. P. & Porter, N. A. Enantioselective free radical reactions. Acc. Chem. Res. 32, 163–171 (1999).
Brimioulle, R. & Bach, T. Enantioselective Lewis acid catalysis of intramolecular enone [2+2] photocycloaddition reactions. Science 342, 840–843 (2013).
Choi, J. & Fu, G. C. Transition metal-catalyzed alkyl–alkyl bond formation: another dimension in cross-coupling chemistry. Science 356, eaaf7230 (2017).
Fu, G. C. Transition-metal catalysis of nucleophilic substitution reactions: a radical alternative to SN1 and SN2 processes. ACS Cent. Sci. 3, 692–700 (2017).
Cherney, A. H., Kadunce, N. T. & Reisman, S. E. Enantioselective and enantiospecific transition-metal-catalyzed cross-coupling reactions of organometallic reagents to construct C–C bonds. Chem. Rev. 115, 9587–9652 (2015).
Chemler, S. R., Karyakarte, S. D. & Khoder, Z. M. Stereoselective and regioselective synthesis of heterocycles via copper-catalyzed additions of amine derivatives and alcohols to alkenes. J. Org. Chem. 82, 11311–11325 (2017).
Zhu, R. & Buchwald, S. L. Enantioselective functionalization of radical intermediates in redox catalysis: copper-catalyzed asymmetric oxytrifluoromethylation of alkenes. Angew. Chem. Int. Ed. 52, 12655–12658 (2013).
Jiang, H., Lang, K., Lu, H., Wojtas, L. & Zhang, X. P. Asymmetric radical bicyclization of allyl azidoformates via cobalt(ii)-based metalloradical catalysis. J. Am. Chem. Soc. 139, 9164–9167 (2017).
Hao, W., Harenberg, J. H., Wu, X., MacMillan, S. N. & Lin, S. Diastereo- and enantioselective formal [3 + 2] cycloaddition of cyclopropyl ketones and alkenes via Ti-catalyzed radical redox relay. J. Am. Chem. Soc. 140, 3514–3517 (2018).
Beeson, T. D., Mastracchio, A., Hong, J.-B., Ashton, K. & MacMillan, D. W. C. Enantioselective organocatalysis using SOMO activation. Science 316, 582–585 (2007).
Hashimoto, T., Kawamata, Y. & Maruoka, K. An organic thiyl radical catalyst for enantioselective cyclization. Nat. Chem. 6, 702–705 (2014).
Nicewicz, D. A. & MacMillan, D. W. C. Merging photoredox catalysis with organocatalysis: the direct asymmetric alkylation of aldehydes. Science 322, 77–80 (2008).
Arceo, E., Jurberg, I. D., Álvarez-Fernández, A. & Melchiorre, P. Photochemical activity of a key donor–acceptor complex can drive stereoselective catalytic α-alkylation of aldehydes. Nat. Chem. 5, 750–756 (2013).
Rono, L. J., Yayla, H. G., Wang, D. Y., Armstrong, M. F. & Knowles, R. R. Enantioselective photoredox catalysis enabled by proton-coupled electron transfer: development of an asymmetric aza-pinacol cyclization. J. Am. Chem. Soc. 135, 17735–17738 (2013).
Huo, H. et al. Asymmetric photoredox transition-metal catalysis activated by visible light. Nature 515, 100–103 (2014).
Du, J., Skubi, K. L., Schultz, D. M. & Yoon, T. P. A dual-catalysis approach to enantioselective [2 + 2] photocycloadditions using visible light. Science 344, 392–396 (2014).
Proctor, R. S. J., Davis, H. J. & Phipps, R. J. Catalytic enantioselective Minisci-type addition to heteroarenes. Science 360, 419–422 (2018).
Fu, M.-C., Shang, R., Zhao, B., Wang, B. & Fu, Y. Photocatalytic decarboxylative alkylations mediated by triphenylphosphine and sodium iodide. Science 363, 1429–1434 (2019).
Zeng, X.-P., Cao, Z.-Y., Wang, Y.-H., Zhou, F. & Zhou, J. Catalytic enantioselective desymmetrization reactions to all-carbon quaternary stereocenters. Chem. Rev. 116, 7330–7396 (2016).
Saint-Denis, T. G., Zhu, R.-Y., Chen, G., Wu, Q.-F. & Yu, J.-Q. Enantioselective C(sp 3)‒H bond activation by chiral transition metal catalysts. Science 359, eaao4798 (2018).
Metrano, A. J. & Miller, S. J. Peptide-based catalysts reach the outer sphere through remote desymmetrization and atroposelectivity. Acc. Chem. Res. 52, 199–215 (2019).
Ye, K.-Y., McCallum, T. & Lin, S. Bimetallic radical redox-relay catalysis for the isomerization of epoxides to allylic alcohols. J. Am. Chem. Soc. 141, 9548–9554 (2019).
Zhao, Y. & Weix, D. J. Enantioselective cross-coupling of meso-epoxides with aryl halides. J. Am. Chem. Soc. 137, 3237–3240 (2015).
Gansäuer, A., Fan, C.-A., Keller, F. & Karbaum, P. Regiodivergent epoxide opening: a concept in stereoselective catalysis beyond classical kinetic resolutions and desymmetrizations. Chem. Eur. J. 13, 8084–8090 (2007).
Stache, E. E., Rovis, T. & Doyle, A. G. Dual nickel- and photoredox-catalyzed enantioselective desymmetrization of cyclic meso-anhydrides. Angew. Chem. Int. Ed. 56, 3679–3683 (2017).
Bovino, M. T. et al. Enantioselective copper-catalyzed carboetherification of unactivated alkenes. Angew. Chem. Int. Ed. 53, 6383–6387 (2014).
Milan, M., Bietti, M. & Costas, M. Enantioselective aliphatic C–H bond oxidation catalyzed by bioinspired complexes. Chem. Commun. 54, 9559–9570 (2018).
Curran, D. P., Geib, S. J. & Lin, C.-H. Group selective radical cyclizations with Oppolzer’s camphor sultam. Tetrahedron Asymmetry 5, 199–202 (1994).
Villar, F., Kolly-Kovac, T., Equey, O. & Renaud, P. Highly stereoselective radical cyclization of haloacetals controlled by the acetal center. Chem. Eur. J. 9, 1566–1577 (2003).
Kern, N., Plesniak, M. P., McDouall, J. J. W. & Procter, D. J. Enantioselective cyclizations and cyclization cascades of samarium ketyl radicals. Nat. Chem. 9, 1198–1204 (2017).
Zhang, W. et al. Enantioselective cyanation of benzylic C–H bonds via copper-catalyzed radical relay. Science 353, 1014–1018 (2016).
Wang, Z., Yin, H. & Fu, G. C. Catalytic enantioconvergent coupling of secondary and tertiary electrophiles with olefins. Nature 563, 379–383 (2018).
Lin, J.-S. et al. A dual-catalytic strategy to direct asymmetric radical aminotrifluoromethylation of alkenes. J. Am. Chem. Soc. 138, 9357–9360 (2016).
Dong, X.-Y. et al. A general asymmetric copper-catalysed Sonogashira C(sp 3)–C(sp) coupling. Nat. Chem. 11, 1158–1166 (2019).
Gu, Q.-S., Li, Z.-L. & Liu, X.-Y. Copper(I)-catalyzed asymmetric reactions involving radicals. Acc. Chem. Res. 53, 170–181 (2020).
Phipps, R. J., Hamilton, G. L. & Toste, F. D. The progression of chiral anions from concepts to applications in asymmetric catalysis. Nat. Chem. 4, 603–614 (2012).
Parmar, D., Sugiono, E., Raja, S. & Rueping, M. Complete field guide to asymmetric BINOL-phosphate derived Brønsted acid and metal catalysis: history and classification by mode of activation; Brønsted acidity, hydrogen bonding, ion pairing, and metal phosphates. Chem. Rev. 114, 9047–9153 (2014).
Wang, Z., Chen, Z. & Sun, J. Catalytic asymmetric nucleophilic openings of 3-substituted oxetanes. Org. Biomol. Chem. 12, 6028–6032 (2014).
Akiyama, T. & Mori, K. Stronger Brønsted acids: recent progress. Chem. Rev. 115, 9277–9306 (2015).
Kauffman, C. A., Malani, A. N., Easley, C. & Kirkpatrick, P. Posaconazole. Nat. Rev. Drug Discov. 6, 183–184 (2007).
Umezawa, T. Diversity in lignan biosynthesis. Phytochem. Rev. 2, 371–390 (2003).
Xiao, W.-L. et al. Rubriflordilactones A and B, two novel bisnortriterpenoids from Schisandra rubriflora and their biological activities. Org. Lett. 8, 991–994 (2006).
Toti, K. S. et al. Synthesis of an apionucleoside family and discovery of a prodrug with anti-HIV activity. J. Org. Chem. 79, 5097–5112 (2014).
Lorente, A., Lamariano-Merketegi, J., Albericio, F. & Álvarez, M. Tetrahydrofuran-containing macrolides: a fascinating gift from the deep sea. Chem. Rev. 113, 4567–4610 (2013).
Hickman, A. J. & Sanford, M. S. High-valent organometallic copper and palladium in catalysis. Nature 484, 177–185 (2012).
Vogl, E. M., Gröger, H. & Shibasaki, M. Towards perfect asymmetric catalysis: additives and cocatalysts. Angew. Chem. Int. Ed. 38, 1570–1577 (1999).
Hong, L., Sun, W., Yang, D., Li, G. & Wang, R. Additive effects on asymmetric catalysis. Chem. Rev. 116, 4006–4123 (2016).
Cheng, Y.-F., Dong, X.-Y., Gu, Q.-S., Yu, Z.-L. & Liu, X.-Y. Achiral pyridine ligand-enabled enantioselective radical oxytrifluoromethylation of alkenes with alcohols. Angew. Chem. Int. Ed. 56, 8883–8886 (2017).
Quasdorf, K. W. & Overman, L. E. Catalytic enantioselective synthesis of quaternary carbon stereocentres. Nature 516, 181–191 (2014).
Chen, X.-H., Zhang, W.-Q. & Gong, L.-Z. Asymmetric organocatalytic three-component 1,3-dipolar cycloaddition: control of stereochemistry via a chiral Brønsted acid activated dipole. J. Am. Chem. Soc. 130, 5652–5653 (2008).
Ni, C., Hu, M. & Hu, J. Good partnership between sulfur and fluorine: sulfur-based fluorination and fluoroalkylation reagents for organic synthesis. Chem. Rev. 115, 765–825 (2015).
Aho, J. E., Pihko, P. M. & Rissa, T. K. Nonanomeric spiroketals in natural products: structures, sources, and synthetic strategies. Chem. Rev. 105, 4406–4440 (2005).
Charpentier, J., Früh, N. & Togni, A. Electrophilic trifluoromethylation by use of hypervalent iodine reagents. Chem. Rev. 115, 650–682 (2015).
Gephart, R. T. et al. Reaction of CuI with dialkyl peroxides: CuII-alkoxides, alkoxy radicals, and catalytic C–H etherification. J. Am. Chem. Soc. 134, 17350–17353 (2012).
Reid, J. P., Simón, L. & Goodman, J. M. A practical guide for predicting the stereochemistry of bifunctional phosphoric acid catalyzed reactions of imines. Acc. Chem. Res. 49, 1029–1041 (2016).
Duarte, F. & Paton, R. S. Molecular recognition in asymmetric counteranion catalysis: understanding chiral phosphate-mediated desymmetrization. J. Am. Chem. Soc. 139, 8886–8896 (2017).
Wheeler, S. E. Understanding substituent effects in noncovalent interactions involving aromatic rings. Acc. Chem. Res. 46, 1029–1038 (2013).
Lefebvre, C. et al. Accurately extracting the signature of intermolecular interactions present in the NCI plot of the reduced density gradient versus electron density. Phys. Chem. Chem. Phys. 19, 17928–17936 (2017).
Acknowledgements
Financial support from the National Natural Science Foundation of China (21722203 and 21831002 to X.-Y.L., 21702182 and 2187308 to X.H. and 21801116 to Z.-L.L.), Shenzhen Special Funds (JCYJ20170412152435366 and JCYJ20170307105638498 to X.-Y.L.), Shenzhen Nobel Prize Scientists Laboratory Project (C17783101 to X.-Y.L.) and ‘Fundamental Research Funds for the Central Universities’ (2019QNA3009, X.H.) are gratefully acknowledged. Calculations were performed on the high-performance computing system at the Department of Chemistry, Zhejiang University.
Author information
Authors and Affiliations
Contributions
X.-Y.L. conceived of and supervised the project. Y.-F.C., Z.-L.Y., J.W., Q.-S.G. and Z.-L.L. designed the experiments and analysed the data. Y.-F.C., Z.-L.Y., J.W., J.-Q.B., H.-T.W. and X.-J.W. performed the experiments. J.-R.L. and X.H. designed and performed the DFT calculations. X.-Y.L., X.H. and Q.-S.G. wrote the paper. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–9, Tables 1–4, methods and references.
Supplementary Data 1
Cartesian coordinates for calculated species.
Compound 3A′
Crystallographic data for compound 3A′.
Compound 6
Crystallographic data for compound 6.
Compound 8A
Crystallographic data for compound 8A.
Rights and permissions
About this article
Cite this article
Cheng, YF., Liu, JR., Gu, QS. et al. Catalytic enantioselective desymmetrizing functionalization of alkyl radicals via Cu(i)/CPA cooperative catalysis. Nat Catal 3, 401–410 (2020). https://doi.org/10.1038/s41929-020-0439-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-020-0439-8
This article is cited by
-
Catalytic desymmetrization reactions to synthesize all-carbon quaternary stereocentres
Nature Synthesis (2023)
-
Brønsted acid-enhanced copper-catalyzed atroposelective cycloisomerization to axially chiral arylquinolizones via dearomatization of pyridine
Nature Communications (2022)
-
Copper-catalyzed regio- and stereo-selective hydrosilylation of terminal allenes to access (E)-allylsilanes
Nature Communications (2022)
-
Rational enzyme design for enabling biocatalytic Baldwin cyclization and asymmetric synthesis of chiral heterocycles
Nature Communications (2022)
-
Rhodium-catalyzed intermolecular enantioselective Alder–ene type reaction of cyclopentenes with silylacetylenes
Nature Communications (2021)