Abstract
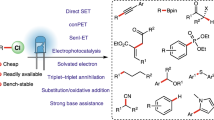
Photoredox catalysis has developed into a powerful tool for the synthesis of organic compounds with diverse structures. However, stable carbon–chloride bonds remain beyond the energetic limits of the outer-sphere photoreductive activation. Here, we demonstrate that the organization of the reacting species in microstructured, aqueous solutions allows generation of carbon-centred radicals from non-activated alkyl chlorides in the presence of double bonds via assembly-promoted single electron transfer. Photocatalytic systems consisting of a surfactant, organic substrates and additives have been designed, characterized and applied for radical dechlorination, addition and cyclization reactions. Cheap and commercially available blue light-emitting diodes are used as the irradiation source for the transformations. Mechanistic studies indicate the accumulation of the energy of two visible light photons in one catalytic cycle.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Data relating to the materials and methods, optimization studies, experimental procedures, mechanistic studies, DLS measurements and NMR spectra are available in the Supplementary Information. All other data are available from the authors upon reasonable request.
References
Clayden, J., Greeves, N. & Warren, S. Organic Chemistry (Oxford Univ. Press, 2012).
Martin, E. T., McGuire, C. M., Mubarak, M. S. & Peters, D. G. Electroreductive remediation of halogenated environmental pollutants. Chem. Rev. 116, 15198–15234 (2016).
Alonso, F., Beletskaya, I. P. & Yus, M. Metal-mediated reductive hydrodehalogenation of organic halides. Chem. Rev. 102, 4009–4091 (2002).
Jefford, C. W., Kirkpatrick, D. & Delay, F. Reductive dehalogenation of alkyl halides with lithium aluminum hydride. A reappraisal of the scope of the reaction. J. Am. Chem. Soc. 94, 8905–8907 (1972).
Carraro, M., Pisano, L. & Azzena, U. Silica gel stabilized Na and Na/K alloys: highly effective, versatile and environmentally friendly reducing agents. Synthesis 49, 1931–1937 (2017).
Dahlén, A., Hilmersson, G., Knettle, B. W. & Flowers, R. A. Rapid SmI2-mediated reductions of alkyl halides and electrochemical properties of SmI2/H2O/amine. J. Org. Chem. 68, 4870–4875 (2003).
Krief, A. & Laval, A.-M. Coupling of organic halides with carbonyl compounds promoted by SmI2, the Kagan reagent. Chem. Rev. 99, 745–778 (1999).
Szostak, M., Spain, M. & Procter, D. J. Determination of the effective redox potentials of SmI2, SmBr2, SmCl2, and their complexes with water by reduction of aromatic hydrocarbons. Reduction of anthracene and stilbene by samarium(II) iodide–water complex. J. Org. Chem. 79, 2522–2537 (2014).
Hammerich, O. & Speiser, B. Organic Electrochemistry: Revised and Expanded 5th edn (CRC Press, 2015).
Kuivila, H. G. Reduction of organic compounds by organotin hydrides. Synthesis 1970, 499–509 (1970).
Kawamoto, T. & Ryu, I. Radical reactions of borohydrides. Org. Biomol. Chem. 12, 9733–9742 (2014).
Neumann, W. P. Tri-n-butyltin hydride as reagent in organic synthesis. Synthesis 1987, 665–683 (1987).
Chatgilialoglu, C., Ferreri, C., Landais, Y. & Timokhin, V. I. Thirty years of (TMS)3SiH: a milestone in radical-based synthetic chemistry. Chem. Rev. 118, 6516–6572 (2018).
Bose, S. K. et al. Highly efficient synthesis of alkylboronate esters via Cu(II)-catalyzed borylation of unactivated alkyl bromides and chlorides in air. ACS Catal. 6, 8–11 (2016).
Atack, T. C. & Cook, S. P. Manganese-catalyzed borylation of unactivated alkyl chlorides. J. Am. Chem. Soc. 138, 6139–6142 (2016).
Frisch, A. C. & Beller, M. Catalysts for cross-coupling reactions with non-activated alkyl halides. Angew. Chem. Int. Ed. Engl. 44, 674–688 (2005).
Hu, X. Nickel-catalyzed cross coupling of non-activated alkyl halides: a mechanistic perspective. Chem. Sci. 2, 1867–1886 (2011).
Dias, H. V. R., Browning, R. G., Polach, S. A., Diyabalanage, H. V. K. & Lovely, C. J. Activation of alkyl halides via a silver-catalyzed carbene insertion process. J. Am. Chem. Soc. 125, 9270–9271 (2003).
Haibach, M. C., Stoltz, B. M. & Grubbs, R. H. Catalytic reduction of alkyl and aryl bromides using propan-2-ol. Angew. Chem. Int. Ed. Engl. 56, 15123–15126 (2017).
Yan, M., Lo, J. C., Edwards, J. T. & Baran, P. S. Radicals: reactive intermediates with translational potential. J. Am. Chem. Soc. 138, 12692–12714 (2016).
Ramaiah, M. Radical reactions in organic synthesis. Tetrahedron 43, 3541–3676 (1987).
Curran, D. P. The design and application of free radical chain reactions in organic synthesis. Part 1. Synthesis 1988, 417–439 (1988).
Jasperse, C. P., Curran, D. P. & Fevig, T. L. Radical reactions in natural product synthesis. Chem. Rev. 91, 1237–1286 (1991).
Giese, B. Preface. Tetrahedron 41, xiii (1985).
Narayanam, J. M. R., Tucker, J. W. & Stephenson, C. R. J. Electron-transfer photoredox catalysis: development of a tin-free reductive dehalogenation reaction. J. Am. Chem. Soc. 131, 8756–8757 (2009).
Neumann, M., Füldner, S., König, B. & Zeitler, K. Metal-free, cooperative asymmetric organophotoredox catalysis with visible light. Angew. Chem. Int. Ed. Engl. 50, 951–954 (2011).
Maji, T., Karmakar, A. & Reiser, O. Visible-light photoredox catalysis: dehalogenation of vicinal dibromo-, α-halo-, and α,α-dibromocarbonyl compounds. J. Org. Chem. 76, 736–739 (2011).
Shimakoshi, H., Tokunaga, M., Baba, T. & Hisaeda, Y. Photochemical dechlorination of DDT catalyzed by a hydrophobic vitamin B12 and a photosensitizer under irradiation with visible light. Chem. Commun. 2004, 1806–1807 (2004).
Tahara, K. & Hisaeda, Y. Eco-friendly molecular transformations catalyzed by a vitamin B12 derivative with a visible-light-driven system. Green Chem. 13, 558–561 (2011).
Tian, H. et al. Photocatalytic function of the B12 complex with the cyclometalated iridium(III) complex as a photosensitizer under visible light irradiation. Dalt. Trans. 47, 675–683 (2018).
Matsubara, R. et al. UVA- and visible-light-mediated generation of carbon radicals from organochlorides using nonmetal photocatalyst. J. Org. Chem. 83, 9381–9390 (2018).
Claros, M. et al. Reductive cyclization of unactivated alkyl chlorides with tethered alkenes under visible-light photoredox catalysis. Angew. Chem. Int. Ed. Engl. 58, 4869–4874 (2019).
Kerzig, C. & Goez, M. Combining energy and electron transfer in a supramolecular environment for the “green” generation and utilization of hydrated electrons through photoredox catalysis. Chem. Sci. 7, 3862–3868 (2016).
Kohlmann, T., Naumann, R., Kerzig, C. & Goez, M. 3-Aminoperylene and ascorbate in aqueous SDS, one green laser flash… and action! Sustainably detoxifying a recalcitrant chloro-organic. Photochem. Photobiol. Sci. 16, 1613–1622 (2017).
Naumann, R., Lehmann, F. & Goez, M. Generating hydrated electrons for chemical syntheses by using a green light-emitting diode (LED). Angew. Chem. Int. Ed. Engl. 57, 1078–1081 (2018).
Naumann, R. & Goez, M. First micelle-free photoredox catalytic access to hydrated electrons for syntheses and remediations with a visible LED or even sunlight. Chem. Eur. J. 24, 17557–17567 (2018).
Kerzig, C., Guo, X. & Wenger, O. S. Unexpected hydrated electron source for preparative visible-light driven photoredox catalysis. J. Am. Chem. Soc. 141, 2122–2127 (2019).
Kohlmann, T., Kerzig, C. & Goez, M. Laser‐induced Wurtz‐type syntheses with a metal‐free photoredox‐catalytic source of hydrated electrons. Chem. Eur. J. 25, 9991–9996 (2019).
Kerzig, C. & Wenger, O. S. Sensitized triplet–triplet annihilation upconversion in water and its application to photochemical transformations. Chem. Sci. 9, 6670–6678 (2018).
Naumann, R. & Goez, M. A green-LED driven source of hydrated electrons characterized from microseconds to hours and applied to cross-couplings. Chem. Eur. J. 24, 9833–9840 (2018).
Meyer, A. U., Slanina, T., Heckel, A. & König, B. Lanthanide ions coupled with photoinduced electron transfer generate strong reduction potentials from visible light. Chem. Eur. J. 23, 7900–7904 (2017).
Häring, M., Pérez-Ruiz, R., Jacobi von Wangelin, A. & Díaz, D. D. Intragel photoreduction of aryl halides by green-to-blue upconversion under aerobic conditions. Chem. Commun. 51, 16848–16851 (2015).
Bardagi, J. I., Ghosh, I., Schmalzbauer, M., Ghosh, T. & König, B. Anthraquinones as photoredox catalysts for the reductive activation of aryl halides. Eur. J. Org. Chem. 2018, 34–40 (2018).
Ghosh, I. & König, B. Chromoselective photocatalysis: Controlled bond activation through light-color regulation of redox potentials. Angew. Chem. Int. Ed. Engl. 55, 7676–7679 (2016).
Ghosh, I., Ghosh, T., Bardagi, J. I. & Konig, B. Reduction of aryl halides by consecutive visible light-induced electron transfer processes. Science 346, 725–728 (2014).
Naumann, R., Lehmann, F. & Goez, M. Micellized tris(bipyridine)ruthenium catalysts affording preparative amounts of hydrated electrons with a green light-emitting diode. Chem. Eur. J. 24, 13259–13269 (2018).
Bauduin, P. et al. The influence of structure and composition of a reverse SDS microemulsion on enzymatic activities and electrical conductivities. J. Colloid Interface Sci. 292, 244–254 (2005).
Devery, J. J., Nguyen, J. D., Dai, C. & Stephenson, C. R. J. Light-mediated reductive debromination of unactivated alkyl and aryl bromides. ACS Catal. 6, 5962–5967 (2016).
Shi, Y., Wu, Y., Hao, J. & Li, G. Microemulsion copolymerization of styrene and acrylonitrile with n-butanol as the cosurfactant. J. Polym. Sci. A 43, 203–216 (2005).
Vlachy, N. et al. Hofmeister series and specific interactions of charged headgroups with aqueous ions. Adv. Colloid Interface Sci. 146, 42–47 (2009).
Tyssee, D. A. & Baizer, M. M. Electrocarboxylation. I. Mono- and dicarboxylation of activated olefins. J. Org. Chem. 39, 2819–2823 (1974).
Kerzig, C., Henkel, S. & Goez, M. A new approach to elucidating repair reactions of resveratrol. Phys. Chem. Chem. Phys. 17, 13915–13920 (2015).
Lambert, F. L. & Ingall, G. B. Voltammetry of organic halogen compounds. IV. The reduction of organic chlorides at the vitreous (glassy) carbon electrode. Tetrahedron Lett. 15, 3231–3234 (1974).
Acknowledgements
We gratefully acknowledge funding from the German Research Foundation (DFG, GRK 1626, Chemical Photocatalysis and KO 1537/18-1) and the Ministry of Science and Higher Education of Poland (M.G., Mobility Plus, 1640/MOB/V/2017/0). We thank S. Crespi for his help with preparing the graphics.
Author information
Authors and Affiliations
Contributions
B.K. guided the research. B.K. and M.G. conceived and designed the project. M.G. performed most of the experiments and wrote the manuscript, with input from others. R.N. expanded the scope of radical cyclization reactions. S.W. carried out part of the optimization experiments. D.T. and W.K. envisaged and interpreted the effects of different surfactants, designed and analysed the DLS experiments. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Tables 1–7, Figs. 1–10 and references.
Rights and permissions
About this article
Cite this article
Giedyk, M., Narobe, R., Weiß, S. et al. Photocatalytic activation of alkyl chlorides by assembly-promoted single electron transfer in microheterogeneous solutions. Nat Catal 3, 40–47 (2020). https://doi.org/10.1038/s41929-019-0369-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-019-0369-5
This article is cited by
-
Energy transfer-mediated multiphoton synergistic excitation for selective C(sp3)–H functionalization with coordination polymer
Nature Communications (2024)
-
Long wavelength near-infrared and red light-driven consecutive photo-induced electron transfer for highly effective photoredox catalysis
Nature Communications (2024)
-
Electrophotocatalytic hydrogenation of imines and reductive functionalization of aryl halides
Nature Communications (2024)
-
Reduction of unactivated alkyl chlorides enabled by light-induced single electron transfer
Science China Chemistry (2024)
-
Formation and degradation of strongly reducing cyanoarene-based radical anions towards efficient radical anion-mediated photoredox catalysis
Nature Communications (2023)